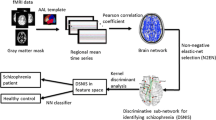
Abstract
Schizophrenia has been proposed to result from impairment of functional connectivity. We aimed to use machine learning to distinguish schizophrenic subjects from normal controls using a publicly available functional MRI (fMRI) data set. Global and local parameters of functional connectivity were extracted for classification. We found decreased global and local network connectivity in subjects with schizophrenia, particularly in the anterior right cingulate cortex, the superior right temporal region, and the inferior left parietal region as compared to healthy subjects. Using support vector machine and 10-fold cross-validation, nine features reached 92.1% prediction accuracy, respectively. Our results suggest that there are significant differences between control and schizophrenic subjects based on regional brain activity detected with fMRI.





Similar content being viewed by others
References
Wei F, Feng W: Research capacity for mental health in low- and middle-income countries: results of a mapping project. Bull World Health Organ 86:908–908, 2008. https://doi.org/10.2471/blt.08.053249
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [Internet]. American Psychiatric Pub; 2013. Available: http://books.google.co.kr/books/about/Diagnostic_and_Statistical_Manual_of_Men.html?hl=&id=-JivBAAAQBAJ
Owen MJ, Akira S, Mortensen PB: Schizophrenia. Lancet 388:86–97, 2016. https://doi.org/10.1016/s0140-6736(15)01121-6
McGlashan TH, Johannessen JO: Early Detection and Intervention With Schizophrenia: Rationale. Schizophr Bull 22:201–222, 1996. https://doi.org/10.1093/schbul/22.2.201
Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA et al.: 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry 155:921–928, 1998. https://doi.org/10.1176/ajp.155.7.921
Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS: The left medial temporal region and schizophrenia. A PET study. Brain 115(Pt 2):367–382, 1992 Available: https://www.ncbi.nlm.nih.gov/pubmed/1606474
Doorduin J, de Vries EFJ, Willemsen ATM, de Groot JC, Dierckx RA, Klein HC: Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 50:1801–1807, 2009. https://doi.org/10.2967/jnumed.109.066647
Kubicki M, McCarley R, Westin C-F, Park H-J, Maier S, Kikinis R et al.: A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res 41:15–30, 2007. https://doi.org/10.1016/j.jpsychires.2005.05.005
Schlösser RGM, Nenadic I, Wagner G, Güllmar D, von Consbruch K, Köhler S et al.: White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res 89:1–11, 2007. https://doi.org/10.1016/j.schres.2006.09.007
Poldrack RA: Region of interest analysis for fMRI. Soc Cogn Affect Neurosci 2:67–70, 2007. https://doi.org/10.1093/scan/nsm006
Zhou Y, Yuan Z, Meng L, Tianzi J, Lixia T, Yong L et al.: Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett 417:297–302, 2007. https://doi.org/10.1016/j.neulet.2007.02.081
Welsh RC, Chen AC, Taylor SF: Low-Frequency BOLD Fluctuations Demonstrate Altered Thalamocortical Connectivity in Schizophrenia. Schizophr Bull 36:713–722, 2008. https://doi.org/10.1093/schbul/sbn145
Wang Y, Yida W, Cohen JD, Kai L, Turk-Browne NB: Full correlation matrix analysis (FCMA): An unbiased method for task-related functional connectivity. J Neurosci Methods 251:108–119, 2015. https://doi.org/10.1016/j.jneumeth.2015.05.012
Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C et al.: Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry 167:343–349, 1995 Available: https://www.ncbi.nlm.nih.gov/pubmed/7496643
Shen H, Wang L, Liu Y, Hu D: Discriminative analysis of resting-state functional connectivity patterns of schizophrenia using low dimensional embedding of fMRI. Neuroimage 49:3110–3121, 2010. https://doi.org/10.1016/j.neuroimage.2009.11.011
Bae Y, Yoo BW, Lee JC, Kim HC: Automated network analysis to measure brain effective connectivity estimated from EEG data of patients with alcoholism. Physiol Meas 38:759–773, 2017. https://doi.org/10.1088/1361-6579/aa6b4c
Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Bradley M et al.: Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology 35:199–210, 1998. https://doi.org/10.1111/1469-8986.3520199
Smith SM, Diego V, Beckmann CF, Glasser MF, Mark J, Miller KL et al.: Functional connectomics from resting-state fMRI. Trends Cogn Sci 17:666–682, 2013. https://doi.org/10.1016/j.tics.2013.09.016
Bush G, George B, Valera EM, Seidman LJ: Functional Neuroimaging of Attention-Deficit/Hyperactivity Disorder: A Review and Suggested Future Directions. Biol Psychiatry 57:1273–1284, 2005. https://doi.org/10.1016/j.biopsych.2005.01.034
Rogers BP, Parks MH, Nickel MK, Katwal SB, Martin PR: Reduced fronto-cerebellar functional connectivity in chronic alcoholic patients. Alcohol Clin Exp Res 36:294–301, 2012. https://doi.org/10.1111/j.1530-0277.2011.01614.x
Allen G, Barnard H, McColl R, Hester AL, Fields JA, Weiner MF et al.: Reduced hippocampal functional connectivity in Alzheimer disease. Arch Neurol 64:1482–1487, 2007. https://doi.org/10.1001/archneur.64.10.1482
Chepenik LG, Mariella R, Michelle H, Cheryl L, Fei W, Jones MM et al.: Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Research: Neuroimaging 182:207–210, 2010. https://doi.org/10.1016/j.pscychresns.2010.04.002
Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR et al.: Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 158:1809–1817, 2001. https://doi.org/10.1176/appi.ajp.158.11.1809
Zarogianni E, Moorhead TWJ, Lawrie SM: Towards the identification of imaging biomarkers in schizophrenia, using multivariate pattern classification at a single-subject level. Neuroimage Clin 3:279–289, 2013. https://doi.org/10.1016/j.nicl.2013.09.003
Zhu M, Jie N, Jiang T: Automatic classification of schizophrenia using resting-state functional language network via an adaptive learning algorithm. SPIE Medical Imaging. Int Soc Optics Photon 903522–903522–6, 2014. doi:https://doi.org/10.1117/12.2043240
Arbabshirani MR, Castro E, Calhoun VD: Accurate classification of schizophrenia patients based on novel resting-state fMRI features. 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE, pp 6691–6694. doi:https://doi.org/10.1109/EMBC.2014.6945163
First MB, Spitzer RL, Gibbon M, Williams JBW, et al: Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. SCID-I/P, 2002
Repovs G, Grega R, Csernansky JG, Barch DM: Brain Network Connectivity in Individuals with Schizophrenia and Their Siblings. Biol Psychiatry 69:967–973, 2011. https://doi.org/10.1016/j.biopsych.2010.11.009
Repovš G, Grega R, Barch DM. Working Memory Related Brain Network Connectivity in Individuals with Schizophrenia and Their Siblings. Front Hum Neurosci. 2012;6. doi:https://doi.org/10.3389/fnhum.2012.00137
Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE: Statistical Parametric Mapping: The Analysis of Functional Brain Images [Internet]. Academic Press, 2011. Available: http://books.google.co.kr/books/about/Statistical_Parametric_Mapping_The_Analy.html?hl=&id=G_qdEsDlkp0C
Ridgway GR, Omar R, Ourselin S, Hill DLG, Warren JD, Fox NC: Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage 44:99–111, 2009. https://doi.org/10.1016/j.neuroimage.2008.08.045
Göttlich M, Martin G, Frederike B, Krämer UM: BASCO: a toolbox for task-related functional connectivity. Front Syst Neurosci 9, 2015. https://doi.org/10.3389/fnsys.2015.00126
Brett M, Anton J-L, Valabregue R, Poline J-B: Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage 16:S497, 2002 Available: http://matthew.dynevor.org/research/abstracts/marsbar/marsbar.pdf
Rousselet GA, Pernet CR: Improving standards in brain-behavior correlation analyses. Front Hum Neurosci 6:119, 2012. https://doi.org/10.3389/fnhum.2012.00119
Pastor-Satorras R, Romualdo P-S, Alexei V, Alessandro V: Dynamical and Correlation Properties of the Internet. Phys Rev Lett 87, 2001. https://doi.org/10.1103/physrevlett.87.258701
Watts DJ, Strogatz SH: Nature 393:440–442, 1998. https://doi.org/10.1038/30918
Freeman LC: Centrality in social networks conceptual clarification. Soc Networks 1:215–239, 1978. https://doi.org/10.1016/0378-8733(78)90021-7
Rubinov M, Sporns O: Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069, 2010. https://doi.org/10.1016/j.neuroimage.2009.10.003
Gao L, Lun G, Taifu L, Lizhong Y, Feng W: Research and application of data mining feature selection based on relief algorithm. J Softw Maint Evol Res Pract 9, 2014. doi:https://doi.org/10.4304/jsw.9.2.515-522
Altman NS: An Introduction to Kernel and Nearest-Neighbor Nonparametric Regression. Am Stat 46:175, 1992. https://doi.org/10.2307/2685209
Meyer D, David M, Friedrich L, Kurt H: The support vector machine under test. Neurocomputing 55:169–186, 2003. https://doi.org/10.1016/s0925-2312(03)00431-4
Surhone LM, Timpledon MT, Marseken SF: Naive Bayes Classifier: Classifier (mathematics), Bayes’ Theorem, Probability Theory, Bayesian Inference, Bayesian Probability, Empirical Bayes Method, Statistics, Conditional Probability [Internet]. 2010. Available: http://books.google.co.kr/books/about/Naive_Bayes_Classifier.html?hl=&id=rIAwQwAACAAJ
Cover TM: Geometrical and Statistical Properties of Systems of Linear Inequalities with Applications in Pattern Recognition. IEEE Trans Comput EC-14:326–334, 1965. https://doi.org/10.1109/pgec.1965.264137
Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AWF, Williams LM, et al: Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. Wiley Online Library 30:403–416, 2009. Available: http://onlinelibrary.wiley.com/doi/10.1002/hbm.20517/full
Heckers S, Goff D, Weiss AP: Reversed hemispheric asymmetry during simple visual perception in schizophrenia. Psychiatry Res 116:25–32, 2002 Available: https://www.ncbi.nlm.nih.gov/pubmed/12426031
Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ: Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage 9:337–342, 1999. https://doi.org/10.1006/nimg.1998.0411
Kim J-J, Kwon JS, Park HJ, Youn T, Kang DH, Kim MS et al.: Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: a [15O] H2O PET study. Am J Psychiatry. Am Psychiatric Assoc 160:919–923, 2003 Available: http://ajp.psychiatryonline.org/doi/abs/10.1176/appi.ajp.160.5.919
Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K et al.: Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 72:5–13, 2015. https://doi.org/10.1001/jamapsychiatry.2014.1734
Yang H, Liu J, Sui J, Pearlson G, Calhoun VD: A Hybrid Machine Learning Method for Fusing fMRI and Genetic Data: Combining both Improves Classification of Schizophrenia. Front Hum Neurosci 4:192, 2010. https://doi.org/10.3389/fnhum.2010.00192
Wang Q, Cheng W, Li M, Ren H, Hu X, Deng W et al.: The CHRM3 gene is implicated in abnormal thalamo-orbital frontal cortex functional connectivity in first-episode treatment-naive patients with schizophrenia. Psychol Med 46:1523–1534, 2016. https://doi.org/10.1017/S0033291716000167
Wong CG, Stevens MC: The Effects of Stimulant Medication on Working Memory Functional Connectivity in Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry 71:458–466, 2012. https://doi.org/10.1016/j.biopsych.2011.11.011
Landin-Romero R, McKenna PJ, Salgado-Pineda P, Sarró S, Aguirre C, Sarri C et al.: Failure of deactivation in the default mode network: a trait marker for schizophrenia? Psychol Med 45:1315–1325, 2014. https://doi.org/10.1017/s0033291714002426
Acknowledgements
This work was funded in part by NCI CA160045.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bae, Y., Kumarasamy, K., Ali, I.M. et al. Differences Between Schizophrenic and Normal Subjects Using Network Properties from fMRI. J Digit Imaging 31, 252–261 (2018). https://doi.org/10.1007/s10278-017-0020-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10278-017-0020-4