Abstract
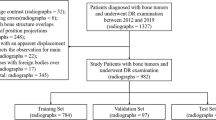
Because many bone tumors have a variety of appearances and are uncommon, few radiologists develop sufficient expertise to guide optimal management. Bayesian inference can guide decision-making by computing probabilities of multiple diagnoses to generate a differential. We built and validated a naïve Bayes machine (NBM) that processes 18 demographic and radiographic features. We reviewed over 1664 analog radiographic cases of bone tumors and selected 811 cases (66 diagnoses) for annotation using a quantitative imaging platform. Leave-one-out cross validation was performed. Primary accuracy was defined as the correct pathological diagnosis as the top machine prediction. Differential accuracy was defined as whether the correct pathological diagnosis was within the top three predictions. For the 29 most common diagnoses (710 cases), primary accuracy was 44%, and differential accuracy was 60%. For the top 10 most common diagnoses (478 cases), primary accuracy was 62%, and differential accuracy was 80%. The machine returned relevant diagnoses for the majority of unknown test cases and may be a feasible alternative to machine learning approaches such as deep neural networks or support vector machines that typically require larger training data (our model required a minimum of five samples per diagnosis) and are “black boxes” (our model can provide details of probability calculations to identify features that most significantly contribute to truth diagnoses). Finally, our Bayes model was designed to scale and “learn” from external data, enabling incorporation of outside knowledge such as Dahlin’s Bone Tumors, a reference of anatomic and demographic statistics of more than 10,000 tumors.



Similar content being viewed by others
References
Costelloe CM, Madewell JE: Radiography in the initial diagnosis of primary bone tumors. AJR Am J Roentgenol 200(1):3–7, 2013
Rajiah P, Ilaslan H, Sundaram M: Imaging of primary malignant bone tumors (nonhematological). Radiol Clin N Am 49(6):1135–1161, 2011 v
Burnside ES: Bayesian networks: computer-assisted diagnosis support in radiology. Acad Radiol 12(4):422–430, 2005
Liu YI et al.: A bayesian network for differentiating benign from malignant thyroid nodules using sonographic and demographic features. AJR Am J Roentgenol 196(5):W598–W605, 2011
Lodwick GS et al.: Computer diagnosis of primary bone tumors: a preliminary report. Radiology 2(80):3, 1963
Lodwick GS: A probabilistic approach to the diagnosis of bone tumors. Radiol Clin N Am 3(3):487–497, 1965
Reinus WR et al.: Diagnosis of focal bone lesions using neural networks. Investig Radiol 29(6):606–611, 1994
Kahn, Jr CE, Laur JJ, Carrera GF: A Bayesian network for diagnosis of primary bone tumors. J Digit Imaging 14(2 Suppl 1):56–57, 2001
Richardson ML: Bayesian bone tumor diagnosis. 2005; Available from: http://uwmsk.org/bayes/bonetumor.html
Doyle LA: Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer 120(12):1763–1774, 2014
Rubin DL et al.: iPad: Semantic annotation and markup of radiological images. AMIA Annu Symp Proc:626–630, 2008
Murphey MD et al.: Enchondroma versus chondrosarcoma in the appendicular skeleton: differentiating features. Radiographics 18(5):1213–1237, 1998 quiz 1244-5
Oudenhoven LF et al.: Accuracy of radiography in grading and tissue-specific diagnosis—a study of 200 consecutive bone tumors of the hand. Skelet Radiol 35(2):78–87, 2006
Miller TT: Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 246(3):662–674, 2008
Cerase A, Priolo F: Skeletal benign bone-forming lesions. Eur J Radiol 27(Suppl 1):S91–S97, 1998
Atesok KI et al.: Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg 19(11):678–689, 2011
Langlotz CP: RadLex: a new method for indexing online educational materials. Radiographics 26(6):1595–1597, 2006
Huynh BQ, Li H, Giger ML: Digital mammographic tumor classification using transfer learning from deep convolutional neural networks. J Med Imaging (Bellingham) 3(3):034501, 2016
Rajkomar A et al.: High-throughput classification of radiographs using deep convolutional neural networks. J Digit Imaging 30(1):95–101, 2017
Kooi T et al.: Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal 35:303–312, 2017
Wang J et al.: Discrimination of breast cancer with microcalcifications on mammography by deep learning. Sci Rep 6:27327, 2016
Lejbkowicz I et al.: Bone browser a decision-aid for the radiological diagnosis of bone tumors. Comput Methods Prog Biomed 67(2):137–154, 2002
Rokach L, Maimon O: Data mining with decision trees: theory and applications. In: Series in Machine Perception and Artificial Intelligence, 2nd edition. Singapore: World Scientific Publishing Company, 2014, p. 328
Unni KK, Inwards CY: Dahlin’s Bone Tumors: General Aspects and Data on 10,165 Cases. Philadelphia: LWW, 2010, p. 416
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional review board approval was obtained. The requirement for informed consent was waived as this was a retrospective review of de-identified radiologic images with only age, gender, and brief clinical notes/diagnosis.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Do, B.H., Langlotz, C. & Beaulieu, C.F. Bone Tumor Diagnosis Using a Naïve Bayesian Model of Demographic and Radiographic Features. J Digit Imaging 30, 640–647 (2017). https://doi.org/10.1007/s10278-017-0001-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10278-017-0001-7