Abstract
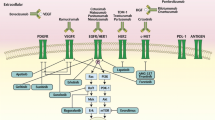
The traditional cytotoxic agents are of limited efficacy in the treatment of well-differentiated endocrine carcinomas (WDEC) of the gastrointestinal tract. WDEC seem to have an extraordinary tumor vascularization with high expression of proangiogenic molecules, such as the vascular endothelial growth factor, along with overexpression of certain tyrosine kinase receptors, such as the epidermal growth factor receptor, the insulin growth factor receptor, and their downstream signaling pathway components (PI3K-AKT-mTOR). Then, molecular targeted therapies seem to be attractive in the treatment of WDEC. We present here recent clinical trials with the first two Phase 3 trials (everolimus or sunitinib versus placebo), positive on progression-free survival, in pancreatic WDEC.
Résumé
La prise en charge thérapeutique des carcinomes endocrines bien différenciés (CEBD) localement avancés ou métastatiques est complexe, pouvant faire appel à de nombreuses armes thérapeutiques (chimiothérapie, immunothérapie, embolisation hépatique, radiothérapie métabolique, etc.), mais reste malheureusement limitée et palliative. Les CEBD présentent la particularité d’avoir un réseau vasculaire très dense, associé à l’hyperexpression de molécules proangiogéniques dont le vascular endothelial growth factor (VEGF). Par ailleurs, l’insulin growth factor receptor (IGF1-R) ou la perte d’antioncogène (PTEN ou NF1), impliqués dans le développement des CEBD, activent la voie PI3/AKT/mTOR. L’utilisation des thérapies ciblant l’angiogenèse et la voie mTOR semble donc attractive pour le traitement des CEBD. Cela a été confirmé récemment pour les CEBD pancréatiques avec un doublement de la survie sans progression dans les deux premières études de phase 3 testant l’everolimus ou le sunitinib versus placebo.
Similar content being viewed by others
Références
Duran I, Kortmansky J, Singh D, et al. (2006) A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer 95: 1148–1154
Durán I, Salazar R, Casanovas O, et al. (2007) New drug development in digestive neuroendocrine tumors. Ann Oncol 18: 1307–1313
Faivre S, Delbaldo C, Vera K, et al. (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine-kinase inhibitor, in patients with cancer. J Clin Oncol 24: 25–35
Hobday TJ, Rubin J, Holen K, et al. (2007) MC044h, a phase II trial of sorafenib in patients (pts) with metastatic neuroendocrine tumors (NET): a phase II consortium (P2C) study. ASCO Meeting Abstracts 25: 4504
Kulke MH, Bergsland EK, Yao JC (2009) Glycemic control in patients with insulinoma treated with everolimus. N Engl J Med 360: 195–197
Kulke MH, Lenz HJ, Meropol NJ, et al. (2008) Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 26: 3403–3410
Kulke MH, Stuart K, Earle CC, et al. (2006) A phase II study of temozolomide and bevacizumab in patients with advanced neuroendocrine tumors. ASCO Meeting Abstracts 24: 4044
Kunz PL, Kuo T, Kaiser HL, et al. (2008) A phase II study of capecitabine, oxaliplatin and bevacizumab for metastatic or unresectable neuroendocrine tumors: preliminary results. ASCO Meeting Abstracts 26: 15502
Mitry E, Ducreux M, Dominguez S, et al. (2009) Bevacizumab combined with chemotherapy in the treatment of advanced/metastatic gastroenteropancreatic tumors: interim safety results from the phase II study. ECCO-ESMO: A6.575
Nicoli P, Raoul J, Bang Y, et al. (2010) Updated safety and efficacy results of the phase III trial of sunitinib versus placebo for treatment of pancreatic neuroendocrine tumors. ASCO Meeting Abstracts 28: 4000
Phan AT, Yao JC, Fogelman DR, et al. (2010) A prospective, multi-institutional phase II study of GW786034 (pazopanib) and depot octreotide (sandostatin LAR) in advanced low-grade neuroendocrine carcinoma (LGNEC). ASCO Meeting Abstracts 28: 4001
Rinke A, Muller HH, Schade-Brittinger C, et al. (2009) Placebo-controlled, double blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27: 4656–4663
Solcia E, Klöppel G, Sobin LH (2000) World Health Organization international classification of tumors. Histological typing of endocrine tumors. Springer-Verlag, New York
Venook AP, Ko AH, Tempero MA, et al. (2008) Phase II trial of Folfox plus bevacizumab in advanced, progressive neuroendocrine tumors. ASCO Meeting Abstracts 26: 15545
Villaume K, Blanc M, Gouysse G, et al. (2010) VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology 91: 268–278
Vinik A, Bang Y, Raoul J, et al. (2010) Patient-reported outcomes in patients with neuroendocrine tumors receiving sunitinib in a phase III trial. ASCO Meeting Abstracts 28: 4003
von Marschall Z, Scholz A, Cramer T, et al. (2003) Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. J Natl Cancer Inst 95: 437–448
Yao JC, Hoff PM (2007) Molecular targeted therapy for neuroendocrine tumors. Hematol Oncol Clin North Am 21: 575–581
Yao JC, Lombard-Bohas C, Baudin E, et al. (2010) Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic. J Clin Oncol 28: 69–76
Yao JC, Phan A, Hoff PM, et al. (2008) Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol 26: 1316–1323
Yao JC, Phan AT, Chang DZ, et al. (2008) Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol 26: 4311–4318
Yao JC, Shah MH, Ito T, et al. (2010) Everolimus versus placebo in patients with advanced pancreatic NET (RADIANT III). 12th WCGI cancer symposium: late breaking abstract
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Walter, T., Lombard-Bohas, C. Apport des antiangiogéniques et des inhibiteurs de mTOR dans le traitement des tumeurs endocrines digestives. Oncologie 12, 636–640 (2010). https://doi.org/10.1007/s10269-010-1947-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10269-010-1947-y