Abstract
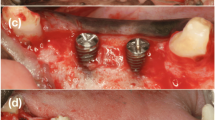
This study compared the in vivo behavior of two biomaterials, xenograft (Bio-Oss®) and alloplastic tricalcium phosphate (Sil-Oss®), vs a control (no biomaterial) in beagle dogs treated with guided bone regeneration (GBR). Six male adult beagle dogs were included. The third and fourth mandibular premolars and first mandibular molars (3P3, 4P4 and 1M1) on both sides were extracted. After 12 weeks of healing, Straumann implants (3.3 × 8 mm) were placed, performing standardized defects (3.3 × 6 mm) in the vestibular aspect of the alveolar bone. The defects were surgically treated by randomized placement of xenograft (Bio-Oss®), alloplastic tricalcium phosphate (Sil-Oss®) or no biomaterial and covered with a resorbable collagen membrane (BioGide®). After an additional 12-week healing period, the lower jaws were dissected. Total area regenerated in the region of interest, total volume, bone to implant contact in the regenerated area, and volumetric changes were measured through histological, histomorphometrical and microcomputed tomography (microCT) techniques. The negative control group showed bone ingrowth inside the defect, with a partial collapse of the buccal bone. This was not observed in the biomaterial-treated groups. Defects treated with the xenograft showed 51.40% (SD 19.83) newly mineralized tissue, while those treated with alloplastic tricalcium showed 62.54% (SD 11.54) newly mineralized tissue; the control showed 71.52% (SD 6.46). Alloplastic tricalcium phosphate modified with monetite and zinc showed similar features in alveolar regeneration of defects to those treated with the xenograft or conventional GBR, but it showed an ideally higher rate of new mineralized tissue formation and accelerated resorption.




Similar content being viewed by others
References
Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25.
Tinti C, Parma-Benfenati S. Clinical classification of bone defects concerning the placement of dental implants. Int J Periodont Restorat Dent. 2003;23:147–55.
Merli M, Merli I, Raffaelli E, Pagliaro U, Nastri L, Nieri M. Bone augmentation at implant dehiscences and fenestrations. A systematic review of randomised controlled trials. Eur J Oral Implantol. 2016;9:11–32.
Hammerle CHF, Tarnow D. The etiology of hard- and soft-tissue deficiencies at dental implants: a narrative review. J Clin Periodontol. 2018;45(2):267–77.
Benic GI, Hammerle CH. Horizontal bone augmentation by means of guided bone regeneration. Periodontol 2000. 2014;66:13–40.
Sanz-Sanchez I, Ortiz-Vigon A, Sanz-Martin I, Figuero E, Sanz M. Effectiveness of lateral bone augmentation on the alveolar crest dimension: a systematic review and meta-analysis. J Dent Res. 2015;94:128–42.
Yamada M, Egusa H. Current bone substitutes for implant dentistry. J Prosthodont Res. 2018;62:152–61.
Miron RJ, Zhang YF. Osteoinduction: a review of old concepts with new standards. J Dent Res. 2012;91:736–44.
Galindo-Moreno P, Moreno-Riestra I, Avila G, et al. Effect of anorganic bovine bone to autogenous cortical bone ratio upon bone remodeling patterns following maxillary sinus augmentation. Clin Oral Implants Res. 2011;22:857–64.
Galindo-Moreno P, Hernandez-Cortes P, Aneiros-Fernandez J, et al. Morphological evidences of Bio-Oss(R) colonization by CD44-positive cells. Clin Oral Implants Res. 2014;25:366–71.
Galindo-Moreno P, Hernandez-Cortes P, Mesa F, et al. Slow resorption of anorganic bovine bone by osteoclasts in maxillary sinus augmentation. Clin Implant Dent Relat Res. 2013;15:858–66.
Galindo-Moreno P, de Buitrago JG, Padial-Molina M, Fernandez-Barbero JE, Ata-Ali J, O Valle F. Histopathological comparison of healing after maxillary sinus augmentation using xenograft mixed with autogenous bone versus allograft mixed with autogenous bone. Clin Oral Implants Res. 2018;29:192–201.
Torres J, Tamimi F, Alkhraisat MH, et al. Vertical bone augmentation with 3D-synthetic monetite blocks in the rabbit calvaria. J Clin Periodontol. 2011;38:1147–53.
Padilla S, de Castro A, Garzón-Gutiérrez A, et al. Novel nanostructured Zn-substituted monetite based biomaterial for bone regeneration. J Nanomed Nanotech. 2015;325:6.
Moonga BS, Dempster DW. Zinc is a potent inhibitor of osteoclastic bone resorption in vitro. J Bone Miner Res. 1995;10:453–7.
Araujo MG, Liljenberg B, Lindhe J. beta-Tricalcium phosphate in the early phase of socket healing: an experimental study in the dog. Clin Oral Implants Res. 2010;21:445–54.
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, National Centre for the Replacement, Refinement and Reduction of Animals in Research. Animal research: reporting in vivo experiments—the ARRIVE guidelines. J Cereb Blood Flow Metab. 2011;31:991–3.
Bruker A. Osteointegration. Analysis of bone around a metal implant (method note No. MN074). 2015. http://www.foa.unesp.br/home/pesquisa/escritorio_de_apoio_a_pesquisa/bruker-micro-ct-academy-2015.pdf. Accessed 17 July 2018.
Larsson L, Decker AM, Nibali L, Pilipchuk SP, Berglundh T, Giannobile WV. Regenerative medicine for periodontal and peri-implant diseases. J Dent Res. 2016;95:255–66.
Galindo-Moreno P, Leon-Cano A, Ortega-Oller I, Monje A, O’Valle F, Catena A. Marginal bone loss as success criterion in implant dentistry: beyond 2 mm. Clin Oral Implants Res. 2015;26:28–34.
Araujo MG, Lindhe J. Ridge alterations following tooth extraction with and without flap elevation: an experimental study in the dog. Clin Oral Implants Res. 2009;20:545–9.
Galindo-Moreno P, Moreno-Riestra I, Avila G, et al. Histomorphometric comparison of maxillary pristine bone and composite bone graft biopsies obtained after sinus augmentation. Clin Oral Implants Res. 2010;21:122–8.
Galindo-Moreno P, Padial-Molina M, Fernandez-Barbero JE, Mesa F, Rodriguez-Martinez D, O’Valle F. Optimal microvessel density from composite graft of autogenous maxillary cortical bone and anorganic bovine bone in sinus augmentation: influence of clinical variables. Clin Oral Implants Res. 2010;21:221–27.
Boeck-Neto RJ, Artese L, Piattelli A, et al. VEGF and MVD expression in sinus augmentation with autologous bone and several graft materials. Oral Dis. 2009;15:148–54.
Marino FT, Torres J, Tresguerres I, Jerez LB, Cabarcos EL. Vertical bone augmentation with granulated brushite cement set in glycolic acid. J Biomed Mater Res A. 2007;81:93–102.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91.
Augustine R, Dominic EA, Reju I, Kaimal B, Kalarikkal N, Thomas S. Electrospun polycaprolactone membranes incorporated with ZnO nanoparticles as skin substitutes with enhanced fibroblast proliferation and wound healing. RSC Adv. 2014;4:24777–85.
Augustine R, Dominic EA, Reju I, Kaimal B, Kalarikkal N, Thomas S. Electrospun poly(epsilon-caprolactone)-based skin substitutes: in vivo evaluation of wound healing and the mechanism of cell proliferation. J Biomed Mater Res B Appl Biomater. 2015;103:1445–54.
Osorio R, Alfonso-Rodriguez CA, Osorio E, et al. Novel potential scaffold for periodontal tissue engineering. Clin Oral Investig. 2017;21:2695–707.
Tamimi FM, Torres J, Tresguerres I, Clemente C, Lopez-Cabarcos E, Blanco LJ. Bone augmentation in rabbit calvariae: comparative study between Bio-Oss and a novel beta-TCP/DCPD granulate. J Clin Periodontol. 2006;33:922–8.
Chou J, Komuro M, Hao J, et al. Bioresorbable zinc hydroxyapatite guided bone regeneration membrane for bone regeneration. Clin Oral Implants Res. 2016;27:354–60.
Flores-Fraile J [Safety and efficacy of the new biomaterial Sil-Oss in the regeneration of post-extraction alveolar ridge. A randomized comparative clinical trial] Universidad de Salamanca. 2016. https://gredos.usal.es/jspui/handle/10366/132812. Accessed 17 July 2018.
Kumar-Deshoju A, Viswa-Chandra R, Amarender-Reddy A, Harish-Reddy B, Nagarajan S, Naveen A. Efficacy of a novel Zn-substituted monetite-based scaffold in the treatment of periodontal osseous. J Intern Acad Periodontol. 2017;19:2–9.
Funding
This study has been partially supported by grants from Ministry of Economy and Competitiveness (Ref. RTC-2014-1731-1). The funders had no role in the study design, data collection and analysis, decision to publish, or in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pérez-Sayáns, M., Lorenzo-Pouso, A.I., Galindo-Moreno, P. et al. Evaluation of a new tricalcium phosphate for guided bone regeneration: an experimental study in the beagle dog. Odontology 107, 209–218 (2019). https://doi.org/10.1007/s10266-018-0384-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-018-0384-z