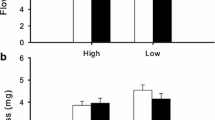
Abstract
Gynodioecy is the coexistence of hermaphrodites and females in a population. It is supposed to be an intermediate stage in the evolutionary pathway from hermaphroditism to dioecy in angiosperm. Hermaphrodites gain fitness through both seed and pollen production whereas females gain fitness only through seed production. As females spread in a gynodioecious population, sexual selection prompts hermaphrodites to invest in male function and male-biased hermaphrodites prevail. In the gynodioecious shrub Daphne jezoensis (Thymelaeaceae), female frequency is stably around 50% in most populations, and fruit-set rate of hermaphrodites is commonly low. Therefore, D. jezoensis is likely at a later stage in the evolutionary pathway. Female function of hermaphrodites (fruit-set rate, selfing rate, seed size, and germination rate) was assessed in three populations under natural conditions. In order to evaluate the potential seed fertility and inbreeding depression by selfing in hermaphrodites, hand pollination treatments were also performed. Over a 2-year period under natural conditions, 18–29% of hermaphrodites and 69–81% of females set fruit. Across all three populations, the mean fruit-set rate ranged 9.5–49.2% in females and only 3.9–10.2% in hermaphrodites. Even with artificial outcross-pollination, 59–91% of hermaphrodites failed to set any fruit. When self-pollination was performed in hermaphrodites, both of fruit-set and germination rates were decreased, indicating early-acting inbreeding depression. In addition, more than half of the hermaphrodite seeds were produced by selfing under natural pollination, but pollinator service was still required. Totally, hermaphrodites performed poorly as seed producers because of the intrinsically-low fruiting ability and a combination of autogamous selfing and strong inbreeding depression, indicating the absence of reproductive assurance. These results indicate that the mating system of D. jezoensis is functionally close to dioecy.




Similar content being viewed by others
References
Ashman T-L (2006) The evolution of separate sexes : a focus on the ecological context. In: Harder L, Barrett SCH (eds) The ecology and evolution of flowers. Oxford University Press, New York, pp 204–222
Bailey MF, Delph LF, Lively CM (2003) Modeling gynodioecy: novel scenarios for maintaining polymorphism. Am Nat 161:762–776
Bell G (1985) On the function of flowers. Proc R Soc B Biol Sci 224:223–265
Busch JW, Delph LF (2012) The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Ann Bot 109:553–562
Charlesworth D (1989) Allocation to male and female function in hermaphrodites, in sexually polymorphic populations. J Theor Biol 139:327–342
Charlesworth B, Charlesworth D (1978) A model for evolution of dioecy and gynodioecy. Am Nat 112:975–997
Charlesworth D, Willis JH (2009) The genetics of inbreeding depression. Nat Rev Genet 10:783–796
Dalton RM, Koski MH, Ashman T-L (2013) Maternal sex effects and inbreeding depression under varied environmental conditions in gynodioecious Fragaria vesca subsp. bracteata. Ann Bot 112:613–621
Del Castillo RF, Argueta ST (2009) Reproductive implications of combined and separate sexes in a trioecious population of Opuntia robusta (Cactaceae). Am J Bot 96:1148–1158
Delph LF (1990) Sex-differential resource allocation patterns in the subdioecious shrub Hebe Subalpina. Ecol Soc Am 71:1342–1351
Delph LF, Wolf DE (2005) Evolutionary consequences of gender plasticity in genetically dimorphic breeding systems. New Phytol 166:119–128
Dufay M, Billard E (2012) How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Ann Bot 109:505–519
Dufay M, Champelovier P, Käfer J, Henry JP, Mousset S, Marais GAB (2014) An angiosperm-wide analysis of the gynodioecy–dioecy pathway. Ann Bot 114:539–548
Eckhart VM (1999) Sexual dimorphism in flowers and inflorescences. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 123–148
Ehlers BK, Bataillon T (2007) “Inconstant males” and the maintenance of labile sex expression in subdioecious plants. New Phytol 174:194–211
Kameyama Y, Hirao AS (2014) Development and evaluation of microsatellite markers for the gynodioecious shrub Daphne jezoensis (Thymelaeaceae). Appl Plant Sci 2:3–5
Kikuzawa K (1989) Floral biology and evolution of gynodioecism in Daphne kamtchatica var. jezoensis. Oikos 56:196–202
Knight T, Steets J, Vamosi J, Mazer S, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman T-L (2005) Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst 36:467–497
Kohn JR (1989) Sex ratio, seed production, biomass allocation, and the cost of male function in Cucurbita foetidissima HBK (Cucurbitaceae). Evolution 43:1424–1434
Larson BM, Barrett SC (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc 69:503–520
Leigh A, Cosgrove MJ, Nicotra AB (2006) Reproductive allocation in a gender dimorphic shrub: anomalous female investment in Gynatrix pulchella? J Ecol 94:1261–1271
Lloyd DG (1976) The transmission of genes via pollen and ovules in gynodioecious angiosperms. Theor Popul Biol 9:299–316
McCauley DE, Brock MT (1998) Frequency-dependent fitness in Silene vulgaris, a gynodioecious plant. Evolution 52:30–36
O’Hara RB, Kotze DJ (2010) Do not log-transform count data. Methods Ecol Evol 1:118–122
Ramsey M, Vaughton G (2002) Maintenance of gynodioecy in Wurmbea biglandulosa (Colchicaceae): gender differences in seed production and progeny success. Plant Syst Evol 232:189–200
Renner SS, Ricklefs RE (1995) Dioecy and its correlates in the flowering plants. Am J Bot 82:596–606
Richards A (1997) Plant breeding systems, 2nd edn. Chapman and Hall, London
Ritland K (1990) Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution 44:1230–1241
Ritland K (2002) Extensions of models for the estimation of mating systems using n independent loci. Heredity 88:221–228
Rousset F (2008) GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour 8:103–106
Sakai AK, Weller SG, Chen M-L, Chou S-Y, Tasanont C (1997) Evolution of gynodioecy and maintenance of females: the role of inbreeding depression, outcrossing rates, and resource allocation in Schiedea adamantis (Caryophyllaceae). Evolution 51:724–736
Shibata A, Kudo G (2017) Size-dependent sex allocation and reproductive investment in a gynodioecious shrub. AoB Plants. doi:10.1093/aobpla/plw089
Shykoff JA, Kolokotronis S-O, Collin CL, López-Villavicencio M (2003) Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia 135:1–9
Sinclair JP, Kameyama Y, Shibata A, Kudo G (2016) Male-biased hermaphrodites in a gynodioecious shrub, Daphne jezoensis. Plant Biol 18:859–867
Spigler RB, Ashman T-L (2012) Gynodioecy to dioecy: are we there yet? Ann Bot 109:531–543
Stewart CN, Via LE (1993) A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. Biotechniques 14:748–750
Van Etten ML, Chang SM (2014) Frequency-dependent pollinator discrimination acts against female plants in the gynodioecious Geranium maculatum. Ann Bot 114:1769–1778
Vaughton G, Ramsey M (2011) Reproductive allocation and costs in gynodioecious Leucopogon melaleucoides (Ericaceae): implications for the evolution of gender dimorphism. Plant Biol 13:888–895
Wang H, Matsushita M, Tomaru N, Nakagawa M (2014) Differences in female reproductive success between female and hermaphrodite individuals in the subdioecious shrub Eurya japonica (Theaceae). Plant Biol 17:194–200
Wolfe LM, Shmida A (1997) The ecology of sex expression in a gynodioecious Israeli desert shrub (Ochradenus Baccatus). Ecology 78:101–110
Acknowledgements
The authors would like to thank Y. Mizunaga, Y. Amagai, K. Onizawa, A. Wakui, S. Nakamura, and T. Kohyama for their kind assistance with fieldwork and their helpful discussions and comments. This study was partly supported by JSPS KAKENHI (15H02641).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shibata, A., Kameyama, Y. & Kudo, G. Restricted female function of hermaphrodites in a gynodioecious shrub, Daphne jezoensis (Thymelaeaceae). J Plant Res 131, 245–254 (2018). https://doi.org/10.1007/s10265-017-0978-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0978-5