Abstract
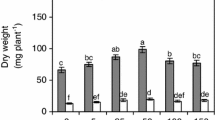
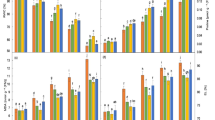
Salinity stress is a major limitation to global crop production. Sugar beet, one of the world’s leading sugar crops, has stronger salt tolerant characteristics than other crops. To investigate the response to different levels of salt stress, sugar beet was grown hydroponically under 3 (control), 70, 140, 210 and 280 mM NaCl conditions. We found no differences in dry weight of the aerial part and leaf area between 70 mM NaCl and control conditions, although dry weight of the root and whole plant treated with 70 mM NaCl was lower than control seedlings. As salt concentrations increased, degree of growth arrest became obvious In addition, under salt stress, the highest concentrations of Na+ and Cl− were detected in the tissue of petioles and old leaves. N and K contents in the tissue of leave, petiole and root decreased rapidly with the increase of NaCl concentrations. P content showed an increasing pattern in these tissues. The activities of antioxidant enzymes such as superoxide dismutase, catalase, ascorbate peroxidase and glutathione peroxidase showed increasing patterns with increase in salt concentrations. Moreover, osmoprotectants such as free amino acids and betaine increased in concentration as the external salinity increased. Two organic acids (malate and citrate) involved in tricarboxylic acid (TCA)-cycle exhibited increasing contents under salt stress. Lastly, we found that Rubisco activity was inhibited under salt stress. The activity of NADP-malic enzyme, NADP-malate dehydrogenase and phosphoenolpyruvate carboxylase showed a trend that first increased and then decreased. Their activities were highest with salinity at 140 mM NaCl. Our study has contributed to the understanding of the sugar beet physiological and metabolic response mechanisms under different degrees of salt stress.







Similar content being viewed by others
References
Aebi H (1984) CAT in vitro. Meth Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Alam MA, Juraimi AS, Rafii MY, Hamid AA, Aslani F, Hakim MA (2016) Salinity-induced changes in the morphology and major mineral nutrient composition of purslane (Portulaca oleracea L.) accessions. Biol Res 49:24. doi:10.1186/s40659-016-0084-5
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341. doi:10.1093/jxb/53.372.1331
Bendaly A, Messedi D, Smaoui A, Ksouri R, Bouchereau A, Abdelly C (2016) Physiological and leaf metabolome changes in the xerohalophyte species Atriplex halimus induced by salinity. Plant Physiol Biochem 103:208–218. doi:10.1016/j.plaphy.2016.02.037
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257. doi:10.1093/jxb/ert430
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448. doi:10.2135/cropsci2005.0437
Cushman JC, Tillett RL, Wood JA, Branco JM, Schlauch KA (2008) Large-scale mRNA expression profiling in the common ice plant, Mesembryanthemum crystallinum performing C3 photosynthesis and crassulacean acid metabolism (CAM). J Exp Bot 59:1875–1894. doi:10.1093/jxb/ern008
Duarte B, Santos D, Marques JC, Caçador I (2013) Ecophysiological adaptations of two halophytes to salt stress: photosynthesis, PS II photochemistry and anti-oxidant feedback–implications for resilience in climate change. Plant Physiol Biochem 67:178–188. doi:10.1016/j.plaphy.2013.03.004
Eschie HA, Al-Barhi B, Al-Gheity S, Al-Khanjari S (2002) Root and shoot growth in salinity-stressed Alfaalfa in respone to nitrogen source. J Plant Nutr 25:2559–2569. doi:10.1081/PLN-120014713
Feller U, Anders I, Mae T (2008) Rubiscolytics: fate of rubisco after its enzymatic function in a cell is terminated. J Exp Bot 59:1615–1624. doi:10.1093/jxb/erm242
Feng K, Yu J, Cheng Y, Ruan M, Wang R, Ye Q, Zhou G, Li Z, Yao Z, Yang Y, Zheng Q, Wan H (2016) The SOD gene family in tomato: identification, phylogenetic relationships, and expression patterns. Front Plant Sci 7:1279. doi:10.3389/fpls.2016.01279
Feria AB, Bosch N, Sánchez A, Nieto-Ingelmo AI, de la Osa C, Echevarría C, García-Mauriño S, Monreal JA (2016) Phosphoenolpyruvate carboxylase (PEPC) and PEPC-kinase (PEPC-k) isoenzymes in Arabidopsis thaliana: role in control and abiotic stress conditions. Planta 244:901–913. doi:10.1007/s00425-016-2556-9
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963. doi:10.1111/j.1469-8137.2008.02531.x
Gawronska K, Romanowska E, Miszalski Z, Niewiadomska E (2013) Limitation of C3-CAM shift in the common ice plant under high irradiance. J Plant Physiol 170:129–135. doi:10.1016/j.jplph.2012.09.019
Hong Z, Lakkineni K, Zhang Z, Verma DP (2000) Removal of feedback inhibition of delta(1)-pyrroline-5-carboxylate synthetase results in increased prolineaccumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136. doi:10.1104/pp.122.4.1129
Hu L, Zhang Z, Xiang Z, Yang Z (2016) Exogenous application of citric acid ameliorates the adverse effect of heat stress in Tall Fescue (Lolium arundinaceum). Front Plant Sci 7:179. doi:10.3389/fpls.2016.00179
Julkowska MM, Testerink C (2015) Tuning plant signaling and growth to survive salt. Trends Plant Sci 20:586–594. doi:10.1016/j.tplants.2015.06.008
Kaur N, Dhawan M, Sharma I, Pati PK (2016) Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC Plant Biol 16:131. doi:10.1186/s12870-016-0824-2
Li W, Wang R, Wang W, Liu H, Liu J, Zhang S, Ai Y (2007) Effect of NaCl stress on sugar beet growth. Sugar Crops China 2:17–19. doi:10.3969/j.issn.1007-2624.2007.02.006
Liu A, Hu Z, Bi A, Fan J, Gitau MM, Amombo E, Chen L, Fu J (2016) Photosynthesis, antioxidant system and gene expression of bermudagrass in response to low temperature and salt stress. Ecotoxicology 25:1445–1457. doi:10.1007/s10646-016-1696-9
Lu R (2000) Soil agricultural chemical analysis method. Agricultural science and technology press, Bei Jing
Luo S, Ishida H, Makino A, Mae T (2002) Fe2+-catalyzed site-specific cleavage of the large subunit of ribulose 1,5-bisphosphate carboxylase close to the active site. J Biol Chem 277:12382–12387. doi:10.1074/jbc.M111072200
Ma D, Guo T, Song X, Wang C, Han Q, Yue Y, Cha F (2010) Effects of nitrogen fertilizer application on RuBP carboxylase activity and chlorophyll fluorescence parameters in flag leaves of winter wheat. Acta Bot Bor Occ Sin 30:2197–2202
Mishra P, Bhoomika K, Dubey RS (2013) Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 250:3–19. doi:10.1007/s00709-011-0365-3
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. doi:10.1016/j.tplants.2004.08.009
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. doi:10.1146/annurev.arplant.59.032607.092911
NakanoY, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. doi:10.1093/oxfordjournals.pcp.a076232
Nisperos-Carriedo MO, Buslig BS, Shaw PE (1992) Simultaneous detection of dehydroascorbic, ascorbic and some organic acids in fruits and vegetables by HPLC. J Agric Food Chem 40:1127–1130. doi:10.1021/jf00019a007
Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12:125–134. doi:10.1016/j.tplants.2007.01.005
Pearce RB, Strange RN, Smith H (1976) Glycinebetaine and choline in wheat: Distribution in relation to infection by Fusarium graminearum. Phytochemistry 15:953–954. doi:10.1016/S0031-9422(00)84377-0
Peng C, Geng G, Yu L, Yang Y, Pi Z, Sun F, Sun X, Zhao H (2014) Effect of different Na+ concentrations on growth and physiological traits of sugar beet. J Plant Nutr Fertil 20:459–465. doi:10.11674/zwyf.2014.0223
Percey WJ, Shabala L, Breadmore MC, Guijt RM, Bose J, Shabala S (2014) Ion transport in broad bean leaf mesophyll under saline conditions. Planta 240:729–743. doi:10.1007/s00425-014-2117-z
Pi Z, Stevanato P, Sun F, Yang Y, Sun X, Zhao H, Geng G, Yu L (2016) Proteomic changes induced by potassium deficiency and potassium substitution by sodium in sugar beet. J Plant Res 129:527–538. doi:10.1007/s10265-016-0800-9
Rahman A, Nahar K, Hasanuzzaman M, Fujita M (2016) Calcium supplementation improves Na(+)/K(+) ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci 7:609. doi:10.3389/fpls.2016.00609
Ren F, Guo QQ, Chang LL, Chen L, Zhao CZ, Zhong H, Li XB (2012) Brassica napus PHR1 gene encoding a MYB-like protein functions in response to phosphate starvation. PLoS One 7:e44005. doi:10.1371/journal.pone.0044005
Robinson MF, Very A, Sanders D, Mansfield TA (1997) How can stomata contribute to salt tolerance? Ann Bot 80:387–393. doi:10.1006/anbo.1996.0435
Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotechnol 26:115–124. doi:10.1016/j.copbio
Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56:1481–1489. doi:10.1093/jxb/eri181
Sharma I, Ching E, Saini S, Bhardwaj R, Pati PK (2013) Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem 69:17–26. doi:10.1016/j.plaphy.2013.04.013
Stewart RR, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65:245–248. doi:10.1104/pp.65.2.245
Tepperman JM, Dunsmuir P (1990) Transformed plants with elevated levels of chloroplastic SOD are not more resistant to superoxide toxicity. Plant Mol Biol 14:501–511. doi:10.1007/BF00027496
Tuteja N (2007) Mechanisms of high salinity tolerance in plants. Meth Enzymol 428:419–438. doi:10.1016/S0076-6879(07)28024-3
Volkmar KM, Hu Y, Steppuhn H (1998) Physiological responses of plants to salinity: a review. Can J Plant Sci 78:19–27. doi:10.4141/P97-020
Wang Y, Zhan Y, Wu C, Gong S, Zhu N, Chen S, Li H (2012) Cloning of a cystatin gene from sugar beet M14 that can enhance plant salt tolerance. Plant Sci 191–192:93–99. doi:10.1016/j.plantsci.2012.05.001
Wang M, Li P, Li C, Pan Y, Jiang X, Zhu D, Zhao Q, Yu J (2014a) SiLEA14, a novel atypical LEA protein, confers abiotic stress resistance in foxtail millet. BMC Plant Biol 14:290. doi:10.1186/s12870-014-0290-7
Wang XY, Wang P, Qi YP, Zhou CP, Yang LT, Liao XY, Wang LQ, Zhu DH, Chen LS (2014b) Effects of granulation on organic acid metabolism and its relation to mineral elements in Citrus grandis juice sacs. Food Chem 145:984–990. doi:10.1016/j.foodchem.2013.09.021
Xu C, Tang X, Shao H, Wang H (2016) Salinity tolerance mechanism of economic halophytes from physiological to molecular hierarchy for improving food quality. Curr Genom 17:207–214. doi:10.2174/1389202917666160202215548
Yang S, Hong N (2012) Improvement on determination of free amino acids content in tea. Food Sci Techol 37:297–305.
Yang L, Zhang Y, Zhu N, Koh J, Ma C, Pan Y, Yu B, Chen S, Li H (2013) Proteomic analysis of salt tolerance in sugar beet monosomic addition line M14. J Prot Res 12:4931–4950. doi:10.1021/pr400177m
Yang C, Zhou Y, Fan J, Fu Y, Shen L, Yao Y, Li R, Fu S, Duan R, Hu X, Guo J (2015) SpBADH of the halophyte Sesuvium portulacastrum strongly confers drought tolerance through ROSscavenging in transgenic Arabidopsis. Plant Physiol Biochem 96:377–387. doi:10.1016/j.plaphy.2015.08.010
Zeng Y, Li L, Yang R, Yi X, Zhang B (2015) Contribution and distribution of inorganic ions and organic compounds to the osmotic adjustment in Halostachys caspica response to salt stress. Sci Rep 5:13639. doi:10.1038/srep13639
Zhang S, Zhang D, Fan S, Du L, Shen Y, Xing L, Li Y, Ma J, Han M (2016) Effect of exogenous GA3 andits inhibitor paclobutrazol on floral formation, endogenous hormones, and flowering-associated genes in ‘Fuji’ apple (Malus domestica Borkh.). Plant Physiol Biochem 107:178–186. doi:10.1016/j.plaphy.2016.06.005
Zhou J, Jiao F, Wu Z, Li Y, Wang X, He X, Zhong W, Wu P (2008) OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol 146:1673–1686. doi:10.1104/pp.107.111443
Acknowledgements
Lisa David from University of Florida is acknowledged for critical reading of the manuscript. This work was supported by the Project of Natural Science Foundation of Heilongjiang province (project no. C2016048: A preliminary study on the molecular drought tolerance mechanism of T510 strains of sugar beet); Project of international Cooperation (project no. 2010DFAN31530: Drought resistance, salt tolerance breeding, and cultivation for energy beet); Project of National Natural Science Foundation of China (project no. 31271779: Excavation of germplasm resources of higher salt tolerance and osmotic regulation mechanism in sugar beet); Youth Science Funds of Heilongjiang University (Project no QL201511: A preliminary study on salt tolerance mechanism of T510 strains of sugar beet).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Stevanato, P., Yu, L. et al. The physiological and metabolic changes in sugar beet seedlings under different levels of salt stress. J Plant Res 130, 1079–1093 (2017). https://doi.org/10.1007/s10265-017-0964-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0964-y