Abstract
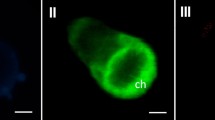
In Chlorophycean algal cells, these organelles are generally called microbodies because they lack the enzymes found in the peroxisomes of higher plants. Microbodies in some algae contain fewer enzymes than the peroxisomes of higher plants, and some unicellular green algae in Chlorophyceae such as Chlamydomonas reinhardtii do not possess catalase, an enzyme commonly found in peroxisomes. Thus, whether microbodies in Chlorophycean algae are similar to the peroxisomes of higher plants, and whether they use a similar transport mechanism for the peroxisomal targeting signal (PTS), remain unclear. To determine whether the PTS is present in the microbodies of Chlorophycean algae, and to visualize the microbodies in Chlamydomonas cells, we examined the sub-cellular localization of green fluorescent proteins (GFP) fused to several PTS-like sequences. We detected GFP compartments that were spherical with a diameter of 0.3–1.0 μm in transgenic Chlamydomonas. Comparative analysis of the character of GFP-compartments observed by fluorescence microscopy and that of microbodies by electron microscopy indicated that the compartments were one and the same. The result also showed that the microbodies in Chlorophycean cells have a similar transport mechanism to that of peroxisomes of higher plants.




Similar content being viewed by others
References
Albertini M, Rehling P, Erdmann R, Girzalsky W, Kiel JA, Veenhuis M, Kunau WH (1997) Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell 89:83–92
Aoyama H, Hagiwara O, Misumi O, Kuroiwa T, Nakamura S (2006) Complete elimination of maternal mitochondrial DNA during meiosis resulting in the paternal inheritance of the mitochondrial genome in Chlamydomonas species. Protoplasma 228:231–242
Baker A (1996) In vitro systems in the study of peroxisomal protein import. Experienti 52:1055–1062
Beevers H (1979) Microbodies in higher plants. Annu Rev Plant Physiol 30:159–197
Beevers H (1982) Glyoxysomes in higher plants. Ann NY Acad Sci 386:243–253
Blattner J, Swinkels B, Dorsam H, Prospero T, Subramani S, Clayton C (1992) Glycosome assembly in trypanosomes:variation in the acceptable degeneracy of a COOH-terminal microbody targeting signal. J Cell Biol 119:1129–1136
De Duve C, Baudhuin P (1966) Peroxisomes (microbodies and related particles). Physiol Rev 46:323–357
Chiu WI, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–330
De Hoop MJ, Ab G (1992) Import of proteins into peroxisomes and other microbodies. Biochem J 286:657–669
Ehara T, Osafune T, Hase E (1995) Behavior of mitochondria in synchronized cells of Chlamydomonas reinhardtii (Chlorophyta). J Cell Sci 108:499–507
Erdmann R, Blobel G (1996) Identification of Pex13p a peroxisomal membrane receptor for the PTS1 recognition factor. J Cell Biol 135:111–121
Frederick SE, Gruber PJ, Tolbert NE (1973) The occurrence of glycolate dehydrogenase and glycolate oxidase in green plants. An evolutionary survey. Plant Physiol 52:318–323
Gietl C (1996) Protein targeting and import into plant peroxisomes. Physiol Plant 97:599–608
Gould SJ, Keller GA, Schneider M, Howell SH, Garrard LJ, Goodman JM, Distel B, Tabak H, Subramani S (1990) Peroxisomal protein import is conserved between yeast, plants, insects and mammals. EMBO J 9:85–90
Hayashi M, Aoki M, Kato A, Kondo M, Nishimura M (1996) Transport of chimeric proteins that contain a carboxy-terminal targeting signal into plant microbodies. Plant J 10:225–234
Hayashi M, Toriyama K, Kondo M, Kato A, Mano S, De Bellis L, Hayashi-Ishimaru Y, Yamaguchi K, Hayashi H, Nishimura M (2000) Functional transformation of plant peroxisomes. Cell Biochem Biophys 32:295–304
Huang AHC, Trelease RN, Moore TS (1983) Plant peroxisomes. Academic Press, New York
Jardim A, Liu W, Zheleznova E, Ullman B (2000) Peroxisomal targeting signal-1 receptor protein PEX5 from Leishmania donovani. J Biol Chem 275:13637–13644
Kato A, Hayashi M, Mori H, Nishimura M (1995) Molecular characterization of a glyoxysomal citrate synthase that is synthesized as a precursor of higher molecular mass in pumpkin. Plant Mol Biol 27:377–390
Kato J, Yamahara T, Tanaka K, Takio S, Satoh T (1997) Characterization of catalase from green algae Chlamydomonas reinhardtii. J Plant Physiol 151:262–268
Kehlenbeck P, Coyal A, Tolbert NE (1995) Factors affecting development of peroxisomes and glycolate metabolism among algae of different evolutionary lines of the Prasinophyceae. Plant Physiol 109:1363–1370
Komori M, Rasmussen SW, Kiel JA, Baerends RJ, Cregg JM, Klei IJV (1997) The Hansenula polymorpha PEX14 gene encodes a novel peroxisomal membrane protein essential for peroxisome biogenesis. EMBO J 16:44–53
Mano S, Nakamori C, Hayashi M, Kato A, Kondo M, Nishimura M (2002) Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: dynamic morphology and actin-dependent movement. Plant Cell Physiol 43:331–341
Marzioch M, Erdmann R, Veenhuis M, Kunau WH (1994) PAS7 encodes a novel yeast member of the WD-40 protein family essential for import of 3-oxoacyl-CoA thiolase, a PTS2-containing protein, into peroxisomes. EMBO J 13:4908–4917
Matsuzaki M, Misumi O, Shin-i T, Maruyama S, Takahara M, Miyagishima S (2004) Genome sequence of the ultra-small unicellular red alga Cyanidioschyzon merolae 10D. Nature 428:653–657
McNew JA, Goodman JM (1996) The targeting and assembly of peroxisomal proteins, some old rules do not apply. Trends Biochem Sci 21:54–58
Motley AM, Hettema EH, Ketting R, Plasterk R, Tabak HF (2000) Caenorhabditis elegans has a single pathway to target matrix proteins to peroxisomes. EMBO Rep 1:40–46
Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H (1999) Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J 18:455–463
Olsen LJ, Harada JJ (1995) Peroxisome and their assembly in higher plants. Annu Rev Plant Physiol Plant Mol Biol 46:123–146
Rhodin J (1954) Correlation of ultrastructural organization and function in normal and experimentally changed proximal convoluted tubule cells of the mouse kidney. Doctorate Thesis. Karolinska Institutet, Stockholm
Shimogawara K, Fujiwara S, Grossman A, Usuda H (1998) High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 148:1821–1828
Shinozaki A, Sato N, Hayashi Y (2009) Peroxisomal targeting signals in green algae. Protoplasma 235:57–66
Silverberg BA (1975a) 3, 3′-Diaminobenzidine (DAB) ultrastructural cytochemistry of microbodies in Chlorogonium elongatum. Protoplasma 85:373–376
Silverberg BA (1975b) An ultrastructural and cytochemical characterization of microbodies in the green algae. Protoplasm 83:269–295
Silverberg BA, Sawa T (1974) Cytochemical localization of oxidase activities with diamino benizidine in the green algae Chlamydomonas dysosmos. Protoplasm 81:177–188
Sizova I, Fuhrmann M, Hegemann PA (2001) Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene 277:221–229
Stabenau H (1974a) Verteilung von Microbody-Enzymen aus Chlamydomonas in Dichtegradienten. Planta 118:35–42
Stabenau H (1974b) Localization of enzymes of the glycolate metabolism in the alga Chlorogonium elongatum Dangeard. Plant Physiol 54:866–869
Stabenau H (1984) Microbodies in different algae. In: Wiessner W, Robinson D, Starr RC (eds) Compartments in algal cells and their interaction. Springer, Berlin, pp 183–190
Stabenau H, Säftel W (1981) Lokalisation von Enzymen des Glykolatstoffwechsels in Mougeotia. Ber Deutsch Bot Ges 94:59–64
Stabenau H, Winkler U, Saftel W (1993) Localization of glycolate dehydrogenase in two species of Dunaliella. Planta 191:362–364
Subramani S (1993) Protein import into peroxisomes and biogenesis of the organelle. Annu Rev Cell Biol 9:445–478
Szilard RK, Titorenko VI, Veenhuis M, Rachubinski RA (1995) Pay32p of the yeast Yarrowia lipolytica is an intraperoxisomal component of the matrix protein translocation machinery. J Cell Biol 131:1453–1469
Terlecky SR, Nuttley WM, McCollum D, Sock E, Subramani S (1995) The Pichia pastoris peroxisomal protein PAS8p is the receptor for the C-terminal tripeptide peroxisomal targeting signal. EMBO J 14:3627–3634
Van der Leij I, Franse MM, Elgersma Y, Distel B, Tabak HF (1993) PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 90:11782–11786
Wimmer C, Schmid M, Veenhuis M, Gietl C (1998) The plant PTS1 receptor: similarities and differences to its human and yeast counterparts. Plant J 16:453–464
Acknowledgments
We thank Dr. Takeshi Ohama of Kochi University of Technology for providing the plasmid pSP103 and Dr Akira Kato of Niigata University for providing cDNA of pumpkin citrate synthase. This work was supported by a Grant for Promotion of Niigata University Research Projects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayashi, Y., Shinozaki, A. Visualization of microbodies in Chlamydomonas reinhardtii . J Plant Res 125, 579–586 (2012). https://doi.org/10.1007/s10265-011-0469-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-011-0469-z