Abstract
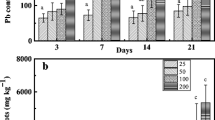
The fern Athyrium yokoscense is known to be highly tolerant to lead toxicity, and is a lead hyperaccumulator that can accumulate over 1,000 μg g−1 of lead in its dry matter. In this work, we examined whether the gametophytic generation of A. yokoscense also resists lead toxicity like the sporophytic generation. Spore germination in A. yokoscense was more tolerant to Pb2+, compared to that in other fern species, such as Pteridium aquilinum, Lygodium japonicum and Pteris vittata. In addition, the early gametophyte development of A. yokoscense was not much affected by 10 μM Pb2+, as evaluated from the prothallial growth and rhizoid development. We also showed that Athyrium gametophytes could accumulate more than 10,000 μg g−1 of lead, and that the lead was localized in the cytosol and vacuole of rhizoidal cells, as determined by a transmission electron micrograph. These results indicate that Athyrium gametophytes have the ability to accumulate lead in the rhizoids. Furthermore, the gametophytes were found to include a large amount of proanthocyanidins (condensed tannins). Because proanthocyanidins have a latent ability to complex with lead ions, the possible roles of proanthocyanidins in the lead tolerance and accumulation of Athyrium gametophytes are discussed.






Similar content being viewed by others
References
Antosiewicz D, Wierzbicka M (1999) Localization of lead in Allium cepa L. cells by electron microscopy. J Microsc 195:139–146
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Banks JA (1999) Gametophyte development in ferns. Annu Rev Plant Physiol Plant Mol Biol 50:163–186
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182 (10.1146/annurev.arplant.53.100301.135154)
Eun S-O, Youn HS, Lee Y (2000) Lead disturbs microtubule organization in the root meristem of Zea mays. Physiol Plant 110:357–365
Francesconi K, Visoottiviseth P, Sridokchan W, Goessler W (2002) Arsenic species in an arsenic hyperaccumulating fern, Pityrogramma calomelanos: a potential phytoremediator of arsenic-contaminated soils. Sci Total Env 284:27–35 (10.1016/S0048-9697(01)00854-3)
Gumaelius L, Lahner B, Salt DE, Banks JA (2004) Arsenic hyperaccumulation in gametophytes of Pteris vittata. A new model system for analysis of arsenic hyperaccumulation. Plant Physiol 136:3198–3208 (10.1104/pp.104.044073)
Gutmann M, Feucht W (1991) A new method for selective localization of flavan-3-ols in plant tissues involving glycolmethacrylate embedding and microwave irradiation. Histochemistry 96:83–86
Hickok LG, Warne TR, Slocum MK (1987) Ceratopteris richardii: applications for experimental plant biology. Am J Bot 74:1304–1316
Ho YB, Tai KM (1985) Potential use of a roadside fern (Pteris vittata) to biomonitor Pb and other aerial metal deposition. Bull Env Contam Toxicol 35:430–438
Honjo T, Suganuma H, Satomi N (1980) The vegetation of the pollution areas caused by the lead tile in Kanazawa Castle. J Phytogeogr Taxon 27:70–73
Honjo T, Hatta A, Taniguchi K (1984) Characterization of heavy metals in indicator plants—studies on the accumulation of lead and tolerance of gregarious fern, Athyrium yokoscense, in the polluted areas from the lead tile of the ruins of Kanazawa Castle, now the campus of Kanazawa University. J Phytogeogr Taxon 32:68–80
Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC (1996) Free histidine as a metal chelator in plants that accumulate nickel. Nature 379:635–638
Lavid N, Schwartz A, Yarden O, Tel-Or E (2001) The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 212:323–331 (10.1007/s004250000400)
Lees GL, Suttill NH, Gruber MY (1993) Condensed tannins in sainfoin. 1. A histological and cytological survey of plant tissues. Can J Bot 71:1147–1152
Lichtenberger O, Neumann D (1997) Analytical electron microscopy as a powerful tool in plant cell biology: examples using electron energy loss spectroscopy and X-ray microanalysis. Eur J Cell Biol 73:378–386
Ma JF (2000) Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol 41:383–390
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic. Nature 409:579
Marles MAS, Ray H, Gruber MY (2003) New perspectives on proanthocyanidin biochemistry and molecular regulation. Phytochemistry 64:367–383 (10.1016/S0031-9422(03)00377-7)
Neumann D, Zur Nieden U, Lichtenberger O, Leopold I (1995) How does Armeria maritima tolerate high heavy metal concentrations? J Plant Physiol 146:704–717
Nishizono H, Ichikawa H, Suzuki S, Ishii F (1987a) The role of the root cell wall in the heavy metal tolerance of Athyrium yokoscense. Plant Soil 101:15–20
Nishizono H, Suzuki S, Ishii F (1987b) Accumulation of heavy metals in the metal-tolerant fern, Athyrium yokoscense, growing on various environments. Plant Soil 102:65–70
Nishizono H, Minemura H, Suzuki S, Ishii F (1988) An inducible copper-thiolate complex in the fern, Athyrium yokoscense: involvement in copper-tolerance of the fern. Plant Cell Physiol 29:1345–1351
Nishizono H, Watanabe T, Orii T, Suzuki S (1989) Suppression of inhibitory effects of copper on enzymatic activities by copper-binding substances from Athyrium yokoscense assayed in vitro. Plant Cell Physiol 30:565–569
Palma G, Freer J, Baeza J (2003) Removal of metal ions by modified Pinus radiata bark and tannins from water solutions. Water Res 37:4974–4980 (10.1016/j.watres.2003.08.008)
Racusen RH (2002) Early development in fern gametophytes: interpreting the transition to prothallial architecture in terms of coordinated photosynthate production and osmotic ion uptake. Ann Bot 89:227–240 (10.1093/aob/mcf032)
Raghavan V (1989) Developmental biology of fern gametophytes. Cambridge University Press, Cambridge
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–668 (10.1146/annurev.arplant.49.1.643)
Sato T, Sakai A (1980) Freezing resistance of gametophytes of the temperate fern, Polystichum retroso-paleaceum. Can J Bot 58:1144–1148
Sato T, Sakai A (1981) Cold tolerance of gametophytes and sporophytes of some cool temperate ferns native to Hokkaido. Can J Bot 59:604–608
Satomi N (1958) On the relation between Athyrium yokoscense and copper mine. J Phytogeogr Taxon 7:26
Singh J, Devi S, Chawla G, Gupta M, Viswanathan PN (1991) Ultrastructural and biochemical effects of cadmium on the aquatic fern Marsilea minuta Linn. Ecotoxicol Env Saf 21:171–181
Smith DL (1972) Staining and osmotic properties of young gametophytes of Polypodium vulgare L. and their bearing on rhizoid function. Protoplasma 74:465–479
Stoutjesdijk PA, Sale PW, Larkin PJ (2001) Possible involvement of condensed tannins in aluminium tolerance of Lotus pedunculatus. Aust J Plant Physiol 28:1063–1074 (10.1071/PP01012)
Sugai M (1999) Developmental biology in fern gametophytes—contributions by Japanese researchers. Plant Morphol 11:32–41
Tomizawa K-I, Manabe K, Sugai M (1982) Changes in phytochrome content during imbibition in spores of the fern Lygodium japonicum. Plant Cell Physiol 23:1305–1308
Tung G, Temple PJ (1996) Histochemical detection of lead in plant tissues. Env Toxicol Chem 15:906–914
Wada M, Kadota A (1989) Photomorphogenesis in lower green plants. Annu Rev Plant Physiol Plant Mol Biol 40:169–191 (10.1146/annurev.pp.40.060189.001125)
Wang J, Zhao F-J, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130:1552–1561 (10.1104/pp.008185)
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. Blackwell Scientific, Oxford
Wenzel WW, Jockwer F (1999) Accumulation of heavy metals in plants grown on mineralised soils of the Austrian Alps. Env Pollut 104:145–155
Wierzbicka M (1998) Lead in the apoplast of Allium cepa root tips: ultrastructural studies. Plant Sci 133:105–119
Wierzbicka M (1999) The effect of lead on the cell cycle in the root meristem of Allium cepa L. Protoplasma 207:186–194
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Xian X (1990) Relationship between distribution of cadmium in root tissue and Cd-tolerance of Athyrium yokoscense. J Env Sci Health A25:621–628
Yoshihara T, Tsunokawa K, Miyano Y, Arashima Y, Hodoshima H, Shoji K, Shimada H, Goto F (2005) Induction of callus from a metal hypertolerant fern, Athyrium yokoscense, and evaluation of its cadmium tolerance and accumulation capacity. Plant Cell Rep 23:579–585 (10.1007/s00299-004-0877-9)
Zhan X-M, Zhao X (2003) Mechanism of lead adsorption from aqueous solutions using an adsorbent synthesized from natural condensed tannin. Water Res 37:3905–3912 (10.1016/S0043-1354(03)00312-9)
Zhang W, Cai Y, Downum KR, Ma LQ (2004) Thiol synthesis and arsenic hyperaccumulation in Pteris vittata (Chinese brake fern). Env Pollut 129:69–78 (10.1016/j.envpol.2003.09.020)
Acknowledgements
We are grateful to Prof. Michizo Sugai (Toyama University) for the kind gift of the spores of Pteridium aquilinum.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamachi, H., Komori, I., Tamura, H. et al. Lead tolerance and accumulation in the gametophytes of the fern Athyrium yokoscense. J Plant Res 118, 137–145 (2005). https://doi.org/10.1007/s10265-005-0202-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-005-0202-x