Abstract
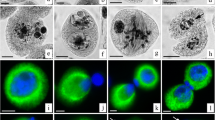
This is the first report on γ-tubulin and microtubule arrays during microsporogenesis in a gymnosperm. Meiosis in Ginkgo biloba is polyplastidic, as is typical of the spermatophyte clade, and microtubule arrays are organized at various sites during meiosis and cytokinesis. In early prophase, a cluster of γ-tubulin globules occurs in the central cytoplasm adjacent to the off-center nucleus. These globules diminish in size and spread over the surface of the nucleus. A system of microtubules focused on the γ-tubulin forms a reticulate pattern in the cytoplasm. As the nucleus migrates to the center of the microsporocyte, γ-tubulin becomes concentrated at several sites adjacent to the nuclear envelope. Microtubules organized at these foci of γ-tubulin give rise to a multipolar prophase spindle. By metaphase I, the spindle has matured into a distinctly bipolar structure with pointed poles. In both first and second meiosis, γ-tubulin becomes distributed throughout the metaphase spindles, but becomes distinctly polar again in anaphase. In telophase I, γ-tubulin moves from polar regions to the proximal surface of chromosome groups/nuclei where interzonal microtubules are organized. No cell wall is deposited and the interzonal microtubules embrace a plate of organelles between the two nuclear cytoplasmic domains (NCDs) of the dyad. Following second meiosis, phragmoplasts that form between sister and non-sister nuclei fuse to form a complex six-sided structure that directs simultaneous cytokinesis. γ-Tubulin becomes associated with nuclei after both meiotic divisions and is especially conspicuous in the distal hemisphere of each young microspore where an unusual encircling system of cortical microtubules develops.



Similar content being viewed by others
References
Barnes SH, Blackmore S (1986) Some functional features in pollen development. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic, London, pp 71–80
Brown RC, Lemmon BE (1987) Division polarity, development, and configuration of microtubule arrays in bryophyte meiosis. I. Meiotic prophase to metaphase I. Protoplasma 137:84–99
Brown RC, Lemmon BE (1991a) The cytokinetic apparatus in meiosis: control of the division plane in the absence of a preprophase band of microtubules. In: Lloyd C (ed) The cytoskeletal basis of plant growth and form. Academic, London, pp 259–273
Brown RC, Lemmon BE (1991b) Plastid polarity and meiotic spindle development in microsporogenesis of Selaginella. Protoplasma 161:168–180
Brown RC, Lemmon BE (1992) Control of division plane in normal and griseofulvin-treated microsporocytes of Magnolia. J Cell Sci 103:1031–1038
Brown RC, Lemmon BE (1993) Diversity of cell division in simple land plants holds clues to evolution of the mitotic and cytokinetic apparatus in higher plants. Mem Torrey Bot Club 25:45–62
Brown RC, Lemmon BE (1995) Methods in plant immunolight microscopy. Methods Cell Biol 49:85–107
Brown RC, Lemmon BE (1997) The quadripolar microtubule system in lower land plants. J Plant Res 110:93–106
Brown RC, Lemmon BE (2001) The cytoskeleton and the spatial control of cytokinesis in the plant life cycle. Protoplasma 215:35–49
Brown RC, Lemmon BE (2004) γ-Tubulin, microtubule arrays, and quadripolarity during sporogenesis in the hepatic Aneura pinguis (L.) Dumort. (Metzgeriales). J Plant Res 117:371–376
Brown RC, Lemmon BE, Nguyen H (2002) The microtubule cycle during successive mitotic waves in the syncytial female gametophyte of ginkgo. J Plant Res 115:491–494
Brown RC, Lemmon BE, Horio T (2004) γ-Tubulin localization changes from discrete polar organizers to anastral spindles and phragmoplasts in mitosis of Marchantia polymorpha L. Protoplasma 224:187–193
Caetano-Pereira CM, Pagliarini MS (2001) A new meiotic abnormality in Zea mays: multiple spindles associated with abnormal cytokinesis in both divisions. Genome 44:865–871
Cowan CR, Carlton PM, Cande WZ (2001) The polar arrangement of telomeres in interphase and meiosis. Rabl organization and the bouquet. Plant Physiol 125:532–538
De Mey J, Lambert A-M, Bajer AS, Moeremans M, De Brabander M (1982) Visualization of microtubules in interphase and mitotic plant cells of Haemanthus endosperm with the immuno-gold staining method. Proc Natl Acad Sci USA 79:1898–1902
Dibbayawan TP, Harper JDI, Marc J (2001) A γ-tubulin antibody against a plant peptide sequence localizes to cell division specific microtubule arrays and organelles in plants. Micron 32:671–678
Franklin AE, Cande WZ (1999) Nuclear organization and chromosome segregation. Plant Cell 11:523–534
Friedman WE, Gifford EM (1997) Development of the male gametophyte of Ginkgo biloba: a window into the reproductive biology of early seed plants. In: Hori T et al (eds) Ginkgo biloba-a global treasure. Springer-Verlag, Tokyo, pp 29–49
Furness CA, Rudall PJ, Sampson FB (2002) Evolution of microsporogenesis in angiosperms. Int J Plant Sci 163:235–260
Hasezawa S, Ueda K, Kumagai K (2000) Time-sequence observations of microtubule dynamics throughout mitosis in living cell suspensions of stable transgenic Arabidopsis-direct evidence for the origin of cortical microtubules at M/G1 interface. Plant Cell Physiol 41:244–250
Hori T, Ridge RW, Tulecke W, Del Tredici P, Trèmouillaux-Guîller J, Tobe H (eds) (1997) Ginkgo biloba: A global treasure. Springer-Verlag, Tokyo
Joshi HC, Palevitz BA (1996) γ-Tubulin and microtubule organization in plants. Trends Cell Biol 6:41–44
Kurmann MH (1990) Exine development in conifers. In: Blackmore S, Knox RB (eds) Microspores: evolution and ontogeny. Academic, London, pp 157–172
Liu B, Marc J, Joshi HC, Palevitz BA (1993) A γ-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci 104:1217–1228
Mann MC (1924) Microsporogenesis of Ginkgo biloba L. with special reference to the distribution of plastids and to cell wall formation. Univ Cal Pub Agr Sci 2:243–248
Otegui MS, Staehelin LA (2004) Electron tomographic analysis of post-meiotic cytokinesis during pollen development in Arabidopsis thaliana. Planta 218:501–515
Ovenchkina Y, Oakley BR (2001) γ-Tubulin in plant cells. Methods Cell Biol 67:195–212
Palevitz B (1993) Morphological plasticity of the mitotic apparatus in plants and its developmental consequences. Plant Cell 5:1001–1009
Pennell RI (1988) Sporogenesis in conifers. Adv Bot Res 15:179–196
Rodkiewicz B, Bednara J, Mostowska A, Duda E, Stobiecka H (1986) The change in disposition of plastids and mitochondria during microsporogenesis and sporogenesis in some higher plants. Acta Bot Neerl 35:209–215
Royer DL, Hickey LJ, Wing SL (2003) Ecological conservatism in the “living fossil” Ginkgo. Paleobiology 29:84–104
Schmit A-C, Stoppin V, Chevrier V, Job D, Lambert A-M (1994) Cell cycle dependent distribution of a centrosomal antigen at the perinuclear MTOC or at the kinetochores of higher plant cells. Chromosoma 103:343–351
Shimamura M, Brown RC, Lemmon BE, Akashi T, Mizuno K, Nishihara N, Tomizawa K-I, Yoshimoto K, Deguchi H, Hosoya H, Horio T, Mineyuki Y (2004) γ-Tubulin in basal land plants: characterization, localization and implication in the evolution of acentriolar microtubule organizing centers. Plant Cell 16:45–59
Staiger CJ, Cande WZ (1990) Microtubule distribution in dv, a maize meiotic mutant defective in the prophase to metaphase transition. Dev Biol 138:231–242
Stoppin V, Vantard M, Schmit A-C, Lambert A-M (1994) Isolated plant nuclei nucleate microtubule assembly: the nuclear surface in higher plants has centrosome-like activity. Plant Cell 6:1099–1106
Vaughn KC, Harper JDI (1998) Microtubule-organizing centers and nucleating sites in land plants. Int Rev Cytol 181:75–149
Vernos I, Karsenti E (1995) Chromosomes take the lead in spindle assembly. Trends Cell Biol 5:297–301
Wolniak SM (1976) Organelle distribution and apportionment during meiosis in the microsporocyte of Ginkgo biloba L. Am J Bot 63:251–258
Acknowledgements
We thank Professor T. Horio, Tokushima University, Japan for the gift of the G9 antibody.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, R.C., Lemmon, B.E. γ-Tubulin and microtubule organization during microsporogenesis in Ginkgo biloba . J Plant Res 118, 121–128 (2005). https://doi.org/10.1007/s10265-005-0199-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-005-0199-1