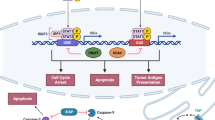
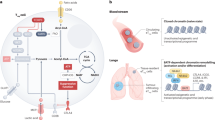
Abstract
Cancer immunotherapy, particularly immune checkpoint inhibitors, has opened a new avenue for cancer treatment following the durable clinical benefits. Despite the clinical successes across several cancer types, primary or acquired resistance might eventually lead to cancer progression in patients with clinical responses. Hence, to broaden the clinical applicability of these treatments, a detailed understanding of the mechanisms limiting the efficacy of cancer immunotherapy is needed. Evidence provided thus far has implicated immunosuppressive innate immune cells infiltrating the tumor microenvironment as key players in immunotherapy resistance. According to the available data, genetic alterations can shape the innate immune response to promote immunotherapy resistance and tumor progression. Herein, this review has discussed the current understanding of the underlying mechanisms where genetic alterations modulate the innate immune milieu to drive immunosuppression and immunotherapy resistance.
Similar content being viewed by others
References
Sun J-Y, Zhang D, Wu S, et al. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives. Biomarker Res. 2020;8(1):35.
Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35.
Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(4):582–8.
Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: implications for immunotherapy. Front Oncol. 2021;11.
Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60.
Wellenstein MD, de Visser KE. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity. 2018;48(3):399–416.
Yang J, Jin L, Kim HS, et al. KDM6A loss recruits tumor-associated neutrophils and promotes neutrophil extracellular trap formation in pancreatic cancer. Can Res. 2022;82(22):4247–60.
Li J, Wang W, Zhang Y, et al. Epigenetic driver mutations in ARID1A shape cancer immune phenotype and immunotherapy. J Clin Invest. 2020;130(5):2712–26.
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23.
Kwantwi LB. Exosome-mediated crosstalk between tumor cells and innate immune cells: implications for cancer progression and therapeutic strategies. J Cancer Res Clin Oncol. 2023.
Kwantwi LB, Wang S, Sheng Y, Wu Q. Multifaceted roles of CCL20 (C-C motif chemokine ligand 20): mechanisms and communication networks in breast cancer progression. Bioengineered. 2021;12(1):6923–34.
Kwantwi LB. The dual and multifaceted role of relaxin-2 in cancer. Clin Transl Oncol. 2023.
Kwantwi LB, Wang S, Zhang W, et al. Tumor-associated neutrophils activated by tumor-derived CCL20 (C-C motif chemokine ligand 20) promote T cell immunosuppression via programmed death-ligand 1 (PD-L1) in breast cancer. Bioengineered. 2021;12(1):6996–7006.
Sheng Y, Peng W, Huang Y, et al. Tumor-activated neutrophils promote metastasis in breast cancer via the G-CSF-RLN2-MMP-9 axis. J Leukoc Biol. 2023;113(4):383–99.
Cai Z, Zhang M, Boafo Kwantwi L, et al. Breast cancer cells promote self-migration by secreting interleukin 8 to induce NET formation. Gene. 2020;754: 144902.
Peng W, Sheng Y, Xiao H, et al. Lung adenocarcinoma cells promote self-migration and self-invasion by activating neutrophils to upregulate notch3 expression of cancer cells. Front Molecul Biosci. 2022;8.
Hagerling C, Casbon AJ, Werb Z. Balancing the innate immune system in tumor development. Trends Cell Biol. 2015;25(4):214–20.
Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18(3):139–47.
Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39.
Ghoneim HE, Zamora AE, Thomas PG, Youngblood BA. Cell-Intrinsic Barriers of T Cell-Based Immunotherapy. Trends Mol Med. 2016;22(12):1000–11.
Siolas D, Vucic E, Kurz E, Hajdu C, Bar-Sagi D. Gain-of-function p53<sup>R172H</sup> mutation drives accumulation of neutrophils in pancreatic tumors, promoting resistance to immunotherapy. Cell reports. 2021;36(8).
Shi Y, Xie T, Wang B, et al. Mutant p53 drives an immune cold tumor immune microenvironment in oral squamous cell carcinoma. Commun Biol. 2022;5(1):757.
Xiao Y, Chen J, Zhou H, et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat Commun. 2022;13(1):758.
Akbay EA, Koyama S, Liu Y, et al. Interleukin-17A Promotes lung tumor progression through neutrophil attraction to tumor sites and mediating resistance to PD-1 blockade. J Thoracic Oncol Off Publicat Int Assoc Study Lung Cancer. 2017;12(8):1268–79.
Liu H, Liang Z, Zhou C, et al. Mutant KRAS triggers functional reprogramming of tumor-associated macrophages in colorectal cancer. Signal Transduct Target Ther. 2021;6(1):144.
Steele CW, Karim SA, Leach JDG, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29(6):832–45.
Liao W, Overman MJ, Boutin AT, et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell. 2019;35(4):559-72.e7.
Zhao Y, Cao Y, Chen Y, et al. B2M gene expression shapes the immune landscape of lung adenocarcinoma and determines the response to immunotherapy. Immunology. 2021;164(3):507–23.
Zhao J, Chen AX, Gartrell RD, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–9.
Wu F, Chen J, Yao K, et al. The infiltration of neutrophil granulocytes due to loss of PTEN was associated with poor response to immunotherapy in renal cell carcinoma. J Inflamm Res. 2022;15:6553–67.
An H-W, Seok SH, Kwon J-W, et al. The loss of epithelial Smad4 drives immune evasion via CXCL1 while displaying vulnerability to combinatorial immunotherapy in gastric cancer. Cell Rep. 2022;41(13): 111878.
Chen X, Gao A, Zhang F, et al. ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation. Theranostics. 2021;11(7):3392–416.
Abril-Rodriguez G, Torrejon DY, Liu W, et al. PAK4 inhibition improves PD-1 blockade immunotherapy. Nature cancer. 2020;1(1):46–58.
Jiang Z, Lim SO, Yan M, et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest. 2021;131(8).
Han H, Jain AD, Truica MI, et al. Small-molecule MYC inhibitors suppress tumor growth and enhance immunotherapy. Cancer Cell. 2019;36(5):483-97.e15.
Morel KL, Sheahan AV, Burkhart DL, et al. EZH2 inhibition activates a dsRNA-STING-interferon stress axis that potentiates response to PD-1 checkpoint blockade in prostate cancer. Nat Cancer. 2021;2(4):444–56.
Su W, Han HH, Wang Y, et al. The polycomb repressor complex 1 drives double-negative prostate cancer metastasis by coordinating stemness and immune suppression. Cancer Cell. 2019;36(2):139-55.e10.
Zhu Y, Zhao Y, Wen J, et al. Targeting the chromatin effector Pygo2 promotes cytotoxic T cell responses and overcomes immunotherapy resistance in prostate cancer. Sci Immunol. 2023;8(81):eade4656.
Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2(1): a001008.
Waarts MR, Stonestrom AJ, Park YC, Levine RL. Targeting mutations in cancer. J Clin Invest. 2022;132(8).
Blagih J, Buck MD, Vousden KH. p53, cancer and the immune response. Journal of cell science. 2020;133(5).
Ghosh M, Saha S, Bettke J, et al. Mutant p53 suppresses innate immune signaling to promote tumorigenesis. Cancer Cell. 2021;39(4):494-508.e5.
Prior IA, Hood FE, Hartley JL. The frequency of ras mutations in cancer. Can Res. 2020;80(14):2969–74.
Nadal E, Chen G, Prensner JR, et al. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thoracic Oncol Off Publicat Int Assoc Study Lung Cancer. 2014;9(10):1513–22.
Campbell JD, Alexandrov A, Kim J, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48(6):607–16.
Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52.
Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7.
Pairawan S, Akcakanat A, Kopetz S, et al. Combined MEK/MDM2 inhibition demonstrates antitumor efficacy in TP53 wild-type thyroid and colorectal cancers with MAPK alterations. Sci Rep. 2022;12(1):1248.
Caetano MS, Zhang H, Cumpian AM, et al. IL6 blockade reprograms the lung tumor microenvironment to limit the development and progression of K-ras–mutant lung cancer. Can Res. 2016;76(11):3189–99.
Sa H, Groß O, Brummer T, Zeiser R. Immune modulatory effects of oncogenic KRAS in cancer. Nat Commun. 2020;11(1):5439.
Polidoro MA, Milana F, Soldani C, et al. Impact of RAS mutations on the immune infiltrate of colorectal liver metastases: a preliminary study. J Leukoc Biol. 2020;108(2):715–21.
Janssen JBE, Medema JP, Gootjes EC, Tauriello DVF, Verheul HMW. Mutant RAS and the tumor microenvironment as dual therapeutic targets for advanced colorectal cancer. Cancer Treat Rev. 2022;109: 102433.
Kwantwi LB. Interplay between tumor-derived factors and tumor-associated neutrophils: opportunities for therapeutic interventions in cancer. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2023.
Kwantwi LB. Overcoming anti-PD-1/PD-L1 immune checkpoint blockade resistance: the role of macrophage, neutrophils and mast cells in the tumor microenvironment. Clinical and Experimental Medicine. 2023.
Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70(1):247–79.
Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):58.
Friedhoff J, Schneider F, Jurcic C, et al. BAP1 and PTEN mutations shape the immunological landscape of clear cell renal cell carcinoma and reveal the intertumoral heterogeneity of T cell suppression: a proof-of-concept study. Cancer Immunol Immunotherapy. 2022.
Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature. 1996;383(6596):168–72.
Dai C, Rennhack JP, Arnoff TE, et al. SMAD4 represses FOSL1 expression and pancreatic cancer metastatic colonization. Cell Rep. 2021;36(4): 109443.
Zhao M, Mishra L, Deng CX. The role of TGF-β/SMAD4 signaling in cancer. Int J Biol Sci. 2018;14(2):111–23.
Wang G, Lu X, Dey P, et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016;6(1):80–95.
Schlessinger J. Receptor tyrosine kinases: legacy of the first two decades. Cold Spring Harbor perspectives in biology. 2014;6(3).
Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(2):S16–22.
Ren X, Li Y, Nishimura C, Zang X. Crosstalk between the B7/CD28 and EGFR pathways: mechanisms and therapeutic opportunities. Genes Dis. 2022;9(5):1181–93.
Peraldo-Neia C, Migliardi G, Mello-Grand M, et al. Epidermal growth factor receptor (EGFR) mutation analysis, gene expression profiling and EGFR protein expression in primary prostate cancer. BMC Cancer. 2011;11(1):31.
Talasila KM, Soentgerath A, Euskirchen P, et al. EGFR wild-type amplification and activation promote invasion and development of glioblastoma independent of angiogenesis. Acta Neuropathol. 2013;125(5):683–98.
Madeddu C, Donisi C, Liscia N, Lai E, Scartozzi M, Macciò A. EGFR-mutated non-small cell lung cancer and resistance to immunotherapy: role of the tumor microenvironment. Int J Mol Sci. 2022;23(12):6489.
Lin Z, Wang Q, Jiang T, Wang W, Zhao JJ. Targeting tumor-associated macrophages with STING agonism improves the antitumor efficacy of osimertinib in a mouse model of EGFR-mutant lung cancer. Frontiers in immunology. 2023;14.
Hao Z, Guo D. EGFR mutation: novel prognostic factor associated with immune infiltration in lower-grade glioma; an exploratory study. BMC Cancer. 2019;19(1):1184.
Yang L, He Y-T, Dong S, et al. Single-cell transcriptome analysis revealed a suppressive tumor immune microenvironment in EGFR mutant lung adenocarcinoma. J Immunother Cancer. 2022;10(2): e003534.
Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4(5):367–73.
Townsley FM, Cliffe A, Bienz M. Pygopus and legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol. 2004;6(7):626–33.
Belenkaya TY, Han C, Standley HJ, et al. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development (Cambridge, England). 2002;129(17):4089–101.
Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8(12):976–90.
Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16(4):253–64.
Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harbor perspectives in medicine. 2014;4(6).
Li M, Fang L, Kwantwi LB, et al. N-Myc promotes angiogenesis and therapeutic resistance of prostate cancer by TEM8. Med Oncol. 2021;38(10):127.
Qiu X, Boufaied N, Hallal T, et al. MYC drives aggressive prostate cancer by disrupting transcriptional pause release at androgen receptor targets. Nat Commun. 2022;13(1):2559.
Kortlever RM, Sodir NM, Wilson CH, et al. Myc cooperates with Ras by programming inflammation and immune suppression. Cell. 2017;171(6):1301-15.e14.
Pello OM, Andrés V. Role of c-MYC in tumor-associated macrophages and cancer progression. Oncoimmunology. 2013;2(2): e22984.
Hadjidaniel MD, Muthugounder S, Hung LT, et al. Tumor-associated macrophages promote neuroblastoma via STAT3 phosphorylation and up-regulation of c-MYC. Oncotarget. 2017;8(53):91516–29.
Dhanasekaran R, Hansen AS, Park J, et al. MYC overexpression drives immune evasion in hepatocellular carcinoma that is reversible through restoration of proinflammatory macrophages. Can Res. 2023;83(4):626–40.
Pasini D, Di Croce L. Emerging roles for polycomb proteins in cancer. Curr Opin Genet Dev. 2016;36:50–8.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13(1):104.
Gan L, Yang Y, Li Q, Feng Y, Liu T, Guo W. Epigenetic regulation of cancer progression by EZH2: from biological insights to therapeutic potential. Biomarker Res. 2018;6(1):10.
Peng D, Kryczek I, Nagarsheth N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249–53.
Ennishi D, Takata K, Béguelin W, et al. Molecular and genetic characterization of MHC deficiency identifies EZH2 as therapeutic target for enhancing immune recognition. Cancer Discov. 2019;9(4):546–63.
Zhu Y, Duong L, Lu X, Lu X. Cancer-cell-intrinsic mechanisms shaping the immunosuppressive landscape of prostate cancer. Asian J Androl. 2023;25(2):171–8.
Dobrinić P, Szczurek AT, Klose RJ. PRC1 drives Polycomb-mediated gene repression by controlling transcription initiation and burst frequency. Nat Struct Mol Biol. 2021;28(10):811–24.
Wang S, C. Ordonez-Rubiano S, Dhiman A, et al. Polycomb group proteins in cancer: multifaceted functions and strategies for modulation. NAR Cancer. 2021;3(4).
Smart SK, Vasileiadi E, Wang X, DeRyckere D, Graham DK. The emerging role of TYRO3 as a therapeutic target in cancer. Cancers (Basel). 2018;10(12).
Dufour F, Silina L, Neyret-Kahn H, et al. TYRO3 as a molecular target for growth inhibition and apoptosis induction in bladder cancer. Br J Cancer. 2019;120(5):555–64.
Hsu P-L, Chien C-W, Tang Y-A, et al. Targeting BRD3 eradicates nuclear TYRO3-induced colorectal cancer metastasis. Sci Adv. 2023;9(15):eade3422.
Park M, Kuen D-S, Park J, et al. TYRO3 blockade enhances anti-PD-1 therapy response by modulating expression of CCN1 in tumor microenvironment. J Immunother Cancer. 2023;11(1): e006084.
Won S-Y, Park J-J, Shin E-Y, Kim E-G. PAK4 signaling in health and disease: defining the PAK4–CREB axis. Exp Mol Med. 2019;51(2):1–9.
Cai S, Ye Z, Wang X, et al. Overexpression of P21-activated kinase 4 is associated with poor prognosis in non-small cell lung cancer and promotes migration and invasion. J Exp Clin Cancer Res. 2015;34(1):48.
Acknowledgements
None.
Funding
No financial support was received for this study.
Author information
Authors and Affiliations
Contributions
LBK conceived the idea, wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Ethical approval and consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kwantwi, L.B. Genetic alterations shape innate immune cells to foster immunosuppression and cancer immunotherapy resistance. Clin Exp Med 23, 4289–4296 (2023). https://doi.org/10.1007/s10238-023-01240-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01240-9