Abstract
Human immunodeficiency virus (HIV) is known to cause hematological malignancy. Hematopoietic stem cell transplantation (HPSCT) is an advanced treatment for that. Currently, there are three successful HIV-eliminated cases, and two received HPSCT from CCR5-absent donors. It is well established that the CCR5 protein on the cell surface assists human immunodeficiency virus entry. Preliminary studies have revealed that knocking out CCR5 and/or CXCR4 may inhibit the viral entry of HIV, which may prove promising in the further development of HIV treatment options. Herein, we suggest performing autologous or allogeneic HSCT with CCR5 KO hematopoietic stem cells in patients who suffer from complicated HIV conditions, particularly drug-resistant HIV or a concurrent diagnosis of HIV with lymphoma/leukemia, to achieve complete HIV remission. Nevertheless, at the clinical forefront of CRISPR-HIV technology, more efforts should be directed to advance nonhuman primate (NHP) models for studies of HIV pathogenesis and off-target assessments within this system.
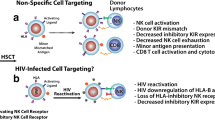
Graphical abstract
CRISPR–Cas9 knock out of host HSCT-expressing CCR5 or CXCR4 may confer HIV-resistance, which when applied to bedside therapeutics in an allogeneic or autologous manner can warrant a permanent and effective treatment outcome.

Similar content being viewed by others
Data availability
Not applicable.
References
Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2:a006866.
Grogg KL, Miller RF, Dogan A. HIV infection and lymphoma. J Clin Pathol. 2007;60:1365–72.
Lurain K, Ramaswami R, Yarchoan R. The role of viruses in HIV-associated lymphomas. Semin Hematol. 2022;59(4):183–91.
Gupta RK, Peppa D, Hill AL, et al. Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV. 2020;7:e340–7.
Freen-van Heeren JJ. Closing the door with CRISPR: genome editing of CCR5 and CXCR4 as a potential curative solution for HIV. BioTech. 2022;11:25.
Wyman C, Kanaar R. DNA double-strand break repair: all’s well that ends well. Annu Rev Genet. 2006;40:363–83.
Kotowski M, Sharma S. CRISPR-based editing techniques for genetic manipulation of primary T cells. Methods Protoc. 2020;3:79.
Ebina H, Misawa N, Kanemura Y, Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3:2510.
Hu W, Kaminski R, Yang F, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci USA. 2014;111:11461–6.
Zhu W, Lei R, Le Duff Y, et al. The CRISPR/Cas9 system inactivates latent HIV-1 proviral DNA. Retrovirology. 2015;12:22.
Wang Z, Pan Q, Gendron P, et al. CRISPR/Cas9-derived mutations both inhibit HIV-1 replication and accelerate viral escape. Cell Rep. 2016;15:481–9.
Liao H-K, Gu Y, Diaz A, et al. Use of the CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells. Nat Commun. 2015;6:6413.
Lebbink RJ, de Jong DCM, Wolters F, et al. A combinational CRISPR/Cas9 gene-editing approach can halt HIV replication and prevent viral escape. Sci Rep. 2017;7:41968.
Lai M, Maori E, Quaranta P, et al. CRISPR/Cas9 ablation of integrated HIV-1 accumulates proviral DNA circles with reformed long terminal repeats. J Virol. 2021;95:e01358-e1421.
Herskovitz J, Hasan M, Patel M, et al. CRISPR–Cas9 mediated exonic disruption for HIV-1 elimination. EBioMedicine. 2021;73:103678.
Weichseldorfer M, Tagaya Y, Reitz M, DeVico AL, Latinovic OS. Identifying CCR5 coreceptor populations permissive for HIV-1 entry and productive infection: implications for in vivo studies. J Transl Med. 2022;20:39.
Perez EE, Wang J, Miller JC, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–16.
Holt N, Wang J, Kim K, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–47.
Wilen CB, Wang J, Tilton JC, et al. Engineering HIV-resistant human CD4+ T cells with CXCR4-specific zinc-finger nucleases. PLoS Pathog. 2011;7:e1002020.
Didigu CA, Wilen CB, Wang J, et al. Simultaneous zinc-finger nuclease editing of the HIV coreceptors ccr5 and cxcr4 protects CD4+ T cells from HIV-1 infection. Blood. 2014;123:61–9.
Cho SW, Kim S, Kim JM, Kim J-S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–2.
Ye L, Wang J, Beyer AI, et al. Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Δ32 mutation confers resistance to HIV infection. Proc Natl Acad Sci USA. 2014;111:9591–6.
Li C, Guan X, Du T, et al. Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirus-delivered CRISPR/Cas9. J Gen Virol. 2015;96:2381–93.
Mandal PK, Ferreira LMR, Collins R, et al. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell. 2014;15:643–52.
Xu L, Yang H, Gao Y, et al. CRISPR/Cas9-mediated CCR5 ablation in human hematopoietic stem/progenitor cells confers HIV-1 resistance in vivo. Mol Ther. 2017;25:1782–9.
Gao Z, Fan M, Das AT, Herrera-Carrillo E, Berkhout B. Extinction of all infectious HIV in cell culture by the CRISPR–Cas12a system with only a single crRNA. Nucleic Acids Res. 2020;48:5527–39.
Yin L, Zhao F, Sun H, et al. CRISPR–Cas13a Inhibits HIV-1 Infection. Mol Ther Nucleic Acids. 2020;21:147–55.
Fan M, Berkhout B, Herrera-Carrillo E. A combinatorial CRISPR–Cas12a attack on HIV DNA. Mol Ther Methods Clin Dev. 2022;25:43–51.
Dash PK, Kaminski R, Bella R, et al. Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat Commun. 2019;10:2753.
Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370:901–10.
Xu L, Wang J, Liu Y, et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med. 2019;381:1240–7.
Jacobson CA, Abramson JS. HIV-associated Hodgkin’s lymphoma: prognosis and therapy in the era of cART. Adv Hematol. 2012;2012:e507257.
Michieli M, Mazzucato M, Tirelli U, De Paoli P. Stem cell transplantation for lymphoma patients with HIV infection. Cell Transpl. 2011;20:351–70.
Al Hamed R, Bazarbachi AH, Malard F, Harousseau J-L, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019;9:44.
Champlin R. Selection of autologous or allogeneic transplantation. In: Holland-Frei Cancer Medicine. 6th edn. 2003. https://www.ncbi.nlm.nih.gov/books/NBK12844/. Accessed 23 Nov 2022.
Grigg A, Ritchie D. Graft-versus-lymphoma effects: clinical review, policy proposals, and immunobiology. Biol Blood Marrow Transpl. 2004;10:579–90.
Scadden DT, Shen H, Cheng T. Hematopoietic stem cells in HIV disease. J Natl Cancer Inst Monogr. 2001;28:24–9.
Cannon P, June C. CCR5 knockout strategies. Curr Opin HIV AIDS. 2011;6:74–9.
Duarte RF, Salgado M, Sánchez-Ortega I, et al. CCR5 Δ32 homozygous cord blood allogeneic transplantation in a patient with HIV: a case report. Lancet HIV. 2015;2:e236-242.
Marx V. The CRISPR children. Nat Biotechnol. 2021;39:1486–90.
Wang G, Zhao N, Berkhout B, Das AT. CRISPR–Cas9 can inhibit HIV-1 replication but NHEJ repair facilitates virus escape. Mol Ther. 2016;24:522–6.
Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38:824–44.
Jensen B-EO, Knops E, Cords L, Lübke N, Salgado M, Busman-Sahay K, Estes JD, Huyveneers LEP, Perdomo-Celis F, Wittner M, Gálvez C, Mummert C, Passaes C, Eberhard JM, Münk C, Hauber I, Hauber J, Heger E, De Clercq J, Vandekerckhove L, Bergmann S, Dunay GA, Klein F, Häussinger D, Fischer JC, Nachtkamp K, Timm J, Kaiser R, Harrer T, Luedde T, Nijhuis M, Sáez-Cirión A, Schulze zur Wiesch J, Wensing AMJ, Martinez-Picado J, Kobbe G. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat Med. 2023;29:583–7.
Doman JL, Raguram A, Newby GA, Liu DR. Evaluation and minimization of Cas9-independent off-target DNA editing by cytosine base editors. Nat Biotechnol. 2020;38:620–8.
Tumwine LK, Orem J, Kerchan P, Byarugaba W, Pileri SA. EBV, HHV8 and HIV in B cell non Hodgkin lymphoma in Kampala, Uganda. Infect Agent Cancer. 2010;5:1–7.
Kashyap R, Rai Mittal B, Manohar K, Balasubramanian Harisankar CN, Bhattacharya A, Singh B, Malhotra P, Varma S. Extranodal manifestations of lymphoma on [18F]FDG-PET/CT: a pictorial essay. Cancer Imaging. 2011;11(1):166–74.
Re A, Cattaneo C, Rossi G. HIV and lymphoma: from epidemiology to clinical management. Mediterr J Hematol Infect Dis. 2019;11(1):e2019004.
Bhatia S. Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for follow-up. Expert Rev Hematol. 2011;4(4):437–52.
Bianchi ME, Mezzapelle R. The chemokine receptor CXCR4 in cell proliferation and tissue regeneration. Front Immunol. 2020;11:2109.
Diaz GA, Gulino AV. WHIM syndrome: a defect in CXCR4 signaling. Curr Allergy Asthma Rep. 2005;5:350–5.
Venuti A, Pastori C, Lopalco L. The role of natural antibodies to CC chemokine receptor 5 in HIV infection. Front Immunol. 2017;8:1358.
Ghorban K, Dadmanesh M, Hassanshahi G, et al. Is the CCR5 Δ 32 mutation associated with immune system-related diseases? Inflammation. 2013;36:633–42.
Tang X, Alatrash G, Ning J, et al. Increasing chimerism following allogeneic stem cell transplantation is associated with longer survival time. Biol Blood Marrow Transpl. 2014;20:1139–44.
Wang L, Wang L, Zhou J, et al. Low-dose decitabine monotherapy reverses mixed chimerism in adult patients after allogeneic hematopoietic stem cell transplantation with myeloablative conditioning regimen: a pilot phase II study. Front Med. 2021;8:627946.
Dai W, Wu F, McMyn N, et al. Genome-wide CRISPR screens identify combinations of candidate latency reversing agents for targeting the latent HIV-1 reservoir. Sci Transl Med. 2022;14:eabh3351.
Hiatt J, Hultquist JF, McGregor MJ, et al. A functional map of HIV-host interactions in primary human T cells. Nat Commun. 2022;13:1752.
Schmidt JK, Reynolds MR, Golos TG, Slukvin II. CRISPR/Cas9 genome editing to create nonhuman primate models for studying stem cell therapies for HIV infection. Retrovirology. 2022;19:17.
Acknowledgements
The graphical abstract was created with Biorender.com.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Search strategy and selection criteria
References for this review were identified through searches on PubMed and CrossRef with defined search terms such as “CRISPR HIV”, “leukemia”, “lymphoma”, “HIV” and “CCR5”. Articles were also identified through searches of the author’s own files, primarily through Google Scholar. Ongoing clinical trials were sourced from ‘ClinicalTrials.gov’. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Au, T.Y., Arudkumar, J., Assavarittirong, C. et al. Killing two birds with one stone: CRISPR/Cas9 CCR5 knockout hematopoietic stem cells transplantation to treat patients with HIV infection and hematological malignancies concurrently. Clin Exp Med 23, 4163–4175 (2023). https://doi.org/10.1007/s10238-023-01129-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01129-7