Abstract
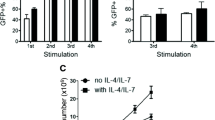
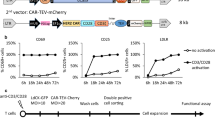
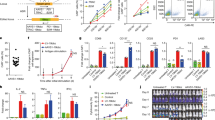
Chimeric antigen receptor T cells (CAR T cells) have improved the prognosis of patients with certain hematologic malignancies. However, broader clinical application of this type of therapy is dependent on production protocols. We characterized VHH-based CD19-redirected CAR T cells generated using the transduction enhancers (TEs) polybrene or retronectin. The proliferation rate of activated T cells transduced using polybrene concentrations > 6 mg/mL decreased compared with untreated group. There was a direct relationship between polybrene concentration and transduction efficacy. Moreover, we demonstrated the proliferation of retronectin-transduced T cells increased in a dose-dependent manner (4–20 μg/mL). Whereas, different retronectin concentrations did not mediate a significant increase in T cell transduction rate. Moreover, lentiviral transduction rate was also dependent on the concentration of lentiviruses. At optimized TE concentrations, multiplicity of infection (MOI) of > 10 decreased living T cell transduction rate. Additionally, we demonstrated that CAR T cell phenotype is highly affected by TE type. Naïve T cell differentiation to central memory T cell was observed in the beginning of the expansion process and effector memory T cells became the predominant subset in the second week of expansion. Importantly, retronectin increased the proliferation of CAR T cells alongside medicating higher transduction rates, resulting in more naïve and central memory T cells. We demonstrated that a higher percentage of CAR T cells were generated using retronectin (with a less differentiated phenotype) making retronectin a more effective TE than polybrene for long-term CAR T cell processing in preclinical or clinical studies.







Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Farkona S, Diamandis EP, Blasutig IMJBM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14(1):1–18.
Murphy A, et al. Gene modification strategies to induce tumor immunity. Immunity. 2005;22(4):403–14.
Rosenberg SA, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308.
Hashem Boroojerdi M, et al. Strategies for having a more effective and less toxic CAR T-cell therapy for acute lymphoblastic leukemia. Med Oncol. 2020;37(11):100.
Safarzadeh Kozani P, Safarzadeh Kozani P, Rahbarizadeh F. Novel antigens of CAR T cell therapy: New roads; old destination. Transl Oncol. 2021;14(7):101079.
Safarzadeh Kozani P, Safarzadeh Kozani P, Rahbarizadeh F. Optimizing the clinical impact of CAR-T cell therapy in B-cell acute lymphoblastic leukemia: looking back while moving forward. Front Immunol. 2021;12:4453.
Safarzadeh Kozani P, et al. Strategies for dodging the obstacles in CAR T cell therapy. Front Oncol. 2021;11(924):627549.
Chang ZL, Chen YYJTIMM. CARs: synthetic immunoreceptors for cancer therapy and beyond. Trends Molecular Med. 2017;23(5):430–50.
Benmebarek M-R, et al. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int J Mol Sci. 2019;20(6):1283.
Zhao J, et al. Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic Res Cardiol. 2002;97(5):348–58.
Milone MC, O’Doherty UJL. Clinical use of lentiviral vectors. Leukemia. 2018;32(7):1529–41.
Bukrinsky MI, et al. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365(6447):666–9.
Tani H, Morikawa S, Matsuura YJFIM. Development and applications of VSV vectors based on cell tropism. Front Microbiol. 2012;2:272.
Levine BL, et al. Global manufacturing of CAR T cell therapy. Molecular Therapy Methods Clin Dev. 2017;4:92–101.
Pizzato M, et al. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J Virol. 1999;73(10):8599–611.
Sharma S, Miyanohara A, Friedmann TJJOV. Separable mechanisms of attachment and cell uptake during retrovirus infection. J Virol. 2000;74(22):10790–5.
Swaney W, et al. The effect of cationic liposome pretreatment and centrifugation on retrovirus-mediated gene transfer. Gene Ther. 1997;4(12):1379–86.
Denning W, et al. Optimization of the transductional efficiency of lentiviral vectors: effect of sera and polycations. Mol Biotechnol. 2013;53(3):308–14.
Guo J, et al. Spinoculation triggers dynamic actin and cofilin activity that facilitates HIV-1 infection of transformed and resting CD4 T cells. J Virol. 2011;85(19):9824–33.
Fratini M, et al. Surface immobilization of viruses and nanoparticles elucidates early events in clathrin-mediated endocytosis. ACS Infect Diseases. 2018;4(11):1585–600.
Sakoda T, et al. A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J Mol Cell Cardiol. 1999;31(11):2037–47.
Schott JW, et al. Enhancing lentiviral and alpharetroviral transduction of human hematopoietic stem cells for clinical application. Molecular Therapy Methods Clin Dev. 2019;14:134–47.
Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nature Rev Clin Oncol. 2018;15(1):31–46.
Taraseviciute A, et al. Chimeric antigen receptor T cell–mediated neurotoxicity in nonhuman primates. Cancer Discov. 2018;8(6):750–63.
Lamers CH, et al. Protocol for gene transduction and expansion of human T lymphocytes for clinical immunogene therapy of cancer. Cancer Gene Therapy. 2002;9(7):613–23.
Costello E, et al. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 2000;7(7):596–604.
Stock S, et al. Influence of retronectin-mediated T-cell activation on expansion and phenotype of CD19-specific chimeric antigen receptor T cells. Hum Gene Ther. 2018;29(10):1167–82.
Yu S, et al. In vivo persistence of genetically modified T cells generated ex vivo using the fibronectin CH296 stimulation method. Cancer Gene Ther. 2008;15(8):508–16.
Gargett T, Brown MPJC. Different cytokine and stimulation conditions influence the expansion and immune phenotype of third-generation chimeric antigen receptor T cells specific for tumor antigen GD2. Cytotherapy. 2015;17(4):487–95.
Ahmadvand D, Rahbarizadeh F, Vishteh VKJH. High-expression of monoclonal nanobodies used in the preparation of HRP-conjugated second antibody. Hybridoma (Larchmt). 2008;27(4):269–76.
Seitz B, et al. Retroviral vector-mediated gene transfer into keratocytes: in vitro effects of polybrene and protamine sulfate. Graefes Arch Clin Exp Ophthalmol. 1998;236(8):602–12.
Lin P, et al. Polybrene inhibits human mesenchymal stem cell proliferation during lentiviral transduction. PLoS ONE. 2011;6(8):e23891.
Poirot L, et al. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Can Res. 2015;75(18):3853–64.
Sharpe M, Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Disease Models Mech. 2015;8(4):337–50.
Safarzadeh Kozani P, Safarzadeh Kozani P, Rahbarizadeh F, Addressing the obstacles of CAR T cell migration in solid tumors: wishing a heavy traffic. Critical Rev Biotechnol, 2022;42(7):1079–1098
Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science Transl Med. 2013;5(177):177ra38.
Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17.
Safarzadeh Kozani P, Safarzadeh Kozani P, Rahbarizadeh F, CAR T cells redirected against tumor-specific antigen glycoforms: can low-sugar antigens guarantee a sweet success? Front Med. 2022;16(3):322–338
Holzinger A, Barden M, Abken HJCI. Immunotherapy, The growing world of CAR T cell trials: a systematic review. Cancer Immunol Immunother. 2016;65(12):1433–50.
Castella M, et al. Point-of-care CAR T-cell production (ARI-0001) using a closed semi-automatic bioreactor: Experience from an academic phase i clinical trial. Front Immunol. 2020;11:482.
Atsavapranee ES, Billingsley MM, Mitchell MJJE. Delivery technologies for T cell gene editing: Applications in cancer immunotherapy. EBioMedicine. 2021;67:103354.
Zhao Y, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13(1):151–9.
Safarzadeh Kozani P, Safarzadeh Kozani P, O’Connor RS. In like a lamb; out like a lion: marching CAR T cells toward enhanced efficacy in B-ALL. Molecular Cancer Ther. 2021;20(7):1223–33.
Harris E, Elmer JJJBP. Optimization of electroporation and other non-viral gene delivery strategies for T cells. Biotechnol Prog. 2021;37(1):e3066.
Singh H, et al. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using sleeping beauty system and artificial antigen presenting cells. PLoS ONE. 2013;8(5):e64138.
Huls MH, et al. Clinical application of sleeping beauty and artificial antigen presenting cells to genetically modify T cells from peripheral and umbilical cord blood. J Vis Exp. 2013;72:e50070.
Lewinski MK, et al. Retroviral DNA integration: viral and cellular determinants of target-site selection. PLoS Pathog. 2006;2(6):e60.
Longo PA, et al. Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol. 2013;529:227–40.
Aubin RJ, et al. Factors influencing efficiency and reproducibility of polybrene-assisted gene transfer. Somat Cell Mol Genet. 1988;14(2):155–67.
Pollok KE, et al. High-efficiency gene transfer into normal and adenosine deaminase-deficient T lymphocytes is mediated by transduction on recombinant fibronectin fragments. J Virol. 1998;72(6):4882–92.
Vorster PJ, et al. LIM kinase 1 modulates cortical actin and CXCR4 cycling and is activated by HIV-1 to initiate viral infection. J Biol Chem. 2011;286(14):12554–64.
Yoder A, et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008;134(5):782–92.
Scanlan PM, et al. Spinoculation of heparan sulfate deficient cells enhances HSV-1 entry, but does not abolish the need for essential glycoproteins in viral fusion. J Virol Methods. 2005;128(1–2):104–12.
O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74(21):10074–80.
Jones KA, Peterlin BM. Control of RNA initiation and elongation at the HIV-1 promoter. Ann Rev Biochem. 1994;63(1):717–43.
Ragheb JA, Deen M, Schwartz RH. CD28-mediated regulation of mRNA stability requires sequences within the coding region of the IL-2 mRNA. J Immunol. 1999;163(1):120–9.
Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172(7):3983–8.
Adomati T, et al. Dead cells induce innate anergy via mertk after acute viral infection. Cell Rep. 2020;30(11):3671–81.
Pay SL, et al. Improving the transduction of bone marrow-derived cells with an integrase-defective lentiviral vector. Human Gene Therapy Methods. 2018;29(1):44–59.
Ghassemi S, et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol Res. 2018;6(9):1100–9.
Tsoukas CD, et al. Activation of resting T lymphocytes by anti-CD3 (T3) antibodies in the absence of monocytes. J Immunol. 1985;135(3):1719–23.
Hosoi H, et al. Stimulation through very late antigen-4 and-5 improves the multifunctionality and memory formation of CD8+ T cells. Eur J Immunol. 2014;44(6):1747–58.
Acknowledgements
This work was partly supported by the National Institute for Medical Research Development (NIMAD) [Grant No. 971379 and 984179], by the Council for Development of Stem Cell Sciences and Technologies [Grant No. 9811642], and by Tarbiat Modares University of Medical Sciences, Tehran, Iran.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
FN: Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—original draft. SM: Formal analysis, Methodology. FR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article. This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was received from all the peripheral blood donors under the guidelines of Tarbiat Modares University Research Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nasiri, F., Muhammadnejad, S. & Rahbarizadeh, F. Effects of polybrene and retronectin as transduction enhancers on the development and phenotypic characteristics of VHH-based CD19-redirected CAR T cells: a comparative investigation. Clin Exp Med 23, 2535–2549 (2023). https://doi.org/10.1007/s10238-022-00928-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00928-8