Abstract
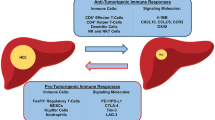
Hepatocellular carcinoma (HCC), a primary malignancy of the liver, is a threat to the health of all humans as a prevalent malignancy and is the sixth most common cancer worldwide. It is difficult to diagnose because symptoms do not show up until late in the disease, and patients often progress to the point where transplantation, resection, or even local treatment cannot be performed. The progression of HCC is regulated by the immune system, and immunotherapy enables the body's immune system's defenses to target liver cancer cells; therefore, immunotherapy has brought a new hope for the treatment of HCC. Currently, the main types of immunotherapies for liver cancer are: immune checkpoint inhibitors, liver cancer vaccines and cellular therapies. In this review, the progress of immunotherapy for the treatment of HCC is summarized.

Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- BCLC:
-
Barcelona Clinical Liver Cancer
- ICIs:
-
Immune checkpoint inhibitors
- ORR:
-
Objective response rate
- mOS:
-
Median overall survival
- mPFS:
-
Median progression-free survival
- mDOR:
-
Median duration remission of response
- TAAs:
-
Tumor-associated antigens
- AFP:
-
Alpha-fetoprotein
- AdV:
-
Replication-deficient adenovirus
- GPC3:
-
Glypican-3
- CTL:
-
T cytotoxicity
- OS:
-
Overall survival
- MRP3:
-
Multidrug resistance-associated protein 3
- hTERT:
-
Human telomerase reverse transcriptase
- CTA:
-
Cancer testicular antigen
- DC:
-
Dendritic cell
- APCs:
-
Antigen-presenting cells
- LNPs:
-
Lipid nanoparticles
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- MHC:
-
Major histocompatibility complex
- PTK:
-
Protein tyrosine kinase
- TPK:
-
Tyrosine protein kinase
- EGFR:
-
Epidermal growth factor receptor
- CRS:
-
Cytokine release syndrome
- TKI:
-
Tyrosine kinase inhibitors
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–91.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
Ferrante ND, Pillai A, Singal AG. Update on the diagnosis and treatment of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2020;16(10):506–16.
Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52(12):1898–907.
Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Ann Rev Pathol Mech Disease. 2021;16:223–49.
Qin SK, Ren ZG, Feng YH, Yau T, Wang BC, Zhao HT, et al. Atezolizumab plus bevacizumab versus sorafenib in the Chinese subpopulation with unresectable hepatocellular carcinoma: phase 3 randomized, open-label IMbrave150 study. Liver Cancer. 2021;10(4):296–308.
Finn RS, Qin SK, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) plus bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021. https://doi.org/10.1200/JCO.2021.39.3_suppl.267.
Zhenggang R, Jianming X, Yuxian B, Aibing X, Shundong C, Chengyou D, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22(7):977–90.
Xu JM, Shen J, Gu SZ, Zhang Y, Wu LH, Wu J, et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A for Nonrandomized, Open-label. Phase II Trial Clin Cancer Res. 2021;27(4):1003–11.
Zhang Y, Xu JM, Shen J, Gu SZ, Wu LH, Wu J, et al. Update on overall survival (OS) of RESCUE: An open-label, phase 2 trial of camrelizumab (C) in combination with apatinib (A) in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2021. https://doi.org/10.1200/JCO.2021.39.15_suppl.4076.
Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS, et al. A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): updated results. Cancer Res. 2019;79(13):v286–7.
Qin S, Chen Z, Liu Y, Xiong J, Zou J, et al. A phase II study of anti–PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J Clin Oncol. 2019. https://doi.org/10.1200/JCO.2019.37.15_suppl.4074.
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu CN, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502.
Za X, Fr S, Julien E, Stéphane C, Sadahisa O, H. PD, et al. Pembrolizumab (pembro) in patients with advanced hepatocellular carcinoma (HCC): KEYNOTE-224 update. J Clin Oncol. 2018. https://doi.org/10.1200/JCO.2018.36.15_suppl.4020.
Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo B-Y, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J Clin Oncol. 2022. https://doi.org/10.1200/JCO.2022.40.4_suppl.383.
Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–80.
Thomas Y, Koo KY, You KT, Anthony BEK, Armando S, Bruno S, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA oncol. 2020;6(11):e204564.
Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022. https://doi.org/10.1200/JCO.2022.40.4_suppl.379.
Zongyi Y, Xiaowu L. Immunotherapy for hepatocellular carcinoma. Cancer Lett. 2020;470:8–17.
Bruno S, Pablo S, Sandra H, Ignacio M. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):525–43.
Luigi B. New vaccination strategies in liver cancer. Cytokine Growth Factor Rev. 2017;36:125–9.
Ma YS, Liu JB, Wu TM, Fu D. New Therapeutic Options for Advanced Hepatocellular Carcinoma. Cancer Control. 2020;27(3):1073274820945975.
Bo L, Qiujiao W, Kun L, Xu D, Mingyue Z, Mengsen L. Alpha-Fetoprotein Binding Mucin and Scavenger Receptors: An Available Bio-Target for Treating Cancer. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.625936.
Christo K, Nikolaos C, Sergios T, Michail V, Dimitrios M, Efthymios G, et al. Immunotherapy for Hepatocellular Carcinoma: a 2021 Update. Cancers (Basel). 2020;12(10):2859.
Masahiro M, Norihiro F, Yasuhiro S, Shoichi M, Keigo S, Toshihiro S, et al. Usefulness of plasma full-length glypican-3 as a predictive marker of hepatocellular carcinoma recurrence after radial surgery. Oncol Lett. 2020;19(4):2657–66.
Li N, Gao W, Zhang Y-F, Ho M, et al. Glypicans as cancer therapeutic targets. Trends Cancer. 2018. https://doi.org/10.1016/j.trecan.2018.09.004.
Yasuhiro S, Toshihiro S, Toshiaki Y, Itaru E, Tetsuya N. Next-Generation Cancer Immunotherapy Targeting Glypican-3. Front Oncol. 2019;9:248.
Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5(5): e1129483.
Rinku D, Mahato RI. Recent advances in hepatocellular carcinoma therapy. Pharmacol Ther. 2017;173:106–17.
Mizukoshi E, Nakagawa H, Kitahara M, Yamashita T, Arai K, Sunagozaka H, et al. Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. Cancer Lett. 2015;369(1):242–9.
Ricardo L, Dias AJ, Donghyun L, Arnaldo F, Uri T, Pedro C-B. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. J Biomed Sci. 2018;25(1):22.
Yongkang Z, Yu-Sheng C, Junzhi Z. Implications of telomerase reverse transcriptase in tumor metastasis. BMB Rep. 2020;53(9):458–65.
Naofumi M, Yasunari N. Emergence of immunotherapy as a novel way to treat hepatocellular carcinoma. World J Gastroenterol. 2018;24(17):1839–58.
Negrini S, Palma RD, Filaci G. Anti-Cancer Immunotherapies Targeting Telomerase. Cancers (Basel). 2020;12(8):2260.
Tim G, Alejandro F, Firouzeh K, Gisele NK, Nathalie B, Carmen A, et al. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10(1):209.
Fabio G, Barbara F, Cody H, Eldo F, Everardo C, Maurizio C-I. Usefulness of cancer-testis antigens as biomarkers for the diagnosis and treatment of hepatocellular carcinoma. J Transl Med. 2007;5(1):3.
Meng W, Jiansheng L, Liping W, Xinfeng C, Zhen Z, Dongli Y, et al. Combined cancer testis antigens enhanced prediction accuracy for prognosis of patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8(4):3513–28.
Baldin AV, Savvateeva LV, Bazhin AV, Zamyatnin AA. Dendritic Cells in Anticancer Vaccination: Rationale for Ex Vivo Loading or In Vivo Targeting. Cancers (Basel). 2020;12(3):590.
Fu YJ, Liu SS, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):396.
Yukio I, Kouichirou T, Shigeru G, Atsushi S, Seiichiro K, Masataka S, et al. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer immunology, immunotherapy : CII. 2003;52(3):155–61.
H PD, S MR, Noweeda M, E TE, Forhad A, C SJ, et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology (Baltimore, Md). 2009;49(1) 124–32.
Qin W, Wei L, Leslie W, Hena K, Won KK, Vadim G, et al. Autologous tumor cell lysate-loaded dendritic cell vaccine inhibited tumor progression in an orthotopic murine model for hepatocellular carcinoma. Ann Surg Oncol. 2016;23(Suppl 5):574–82.
Teng CF, Wang T, Wu TH, Lin JH, Shih FY, Shyu WC, et al. Combination therapy with dendritic cell vaccine and programmed death ligand 1 immune checkpoint inhibitor for hepatocellular carcinoma in an orthotopic mouse model. Ther Adv Med Oncol. 2020;12.
Hobernik D, Bros M. DNA Vaccines—How Far From Clinical Use? Int J Mol Sci. 2018;19(11):3605.
Antitumor immunity induced by DNA vaccine encoding alpha-fetoprotein/heat shock protein 70. World J Gastroenterol. 2004;(21) 3197–200.
Antitumor immunopreventive effect in mice induced by DNA vaccine encoding a fusion protein of α-fetoprotein and CTLA4. World J Gastroenterol. 2004(02):200–4.
H BL, S EJ, Clark GT, A GD. Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients. J Transl Med. 2014;12(1):86.
Lei M, Yu Z, Leaf H. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1).
Yake Z, Fangyuan X, You Y, Qin Z, Hong J, Yan W, et al. Immunotherapy of tumor RNA-loaded lipid nanoparticles against hepatocellular carcinoma. Int J Nanomed. 2021;16:1553–64.
Hammerich L, Binder A, Brody JD. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol Oncol. 2015;9(10):1966–81.
Luciano C, Eleonora A, Giuseppina DA, Laura S, Filippo B. In situ Vaccination by Direct Dendritic Cell Inoculation: The Coming of Age of an Old Idea? Front Immunol. 2019;10:2303.
Anne E, Melissa B, Grant M, Vera K. Simultaneous Tumor and Stroma Targeting by Oncolytic Viruses. Biomedicines. 2020;8(11):274.
Park B-H, Hwang T, Liu T-C, Sze DY, Kim J-S, Kwon H-C, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9(6):533–42.
Ta-Chiang L, Taeho H, Byeong-Ho P, John B, H KD. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16(9) 1637–42.
Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19(3):329–36.
Jeong H, J BC, Anne M, Won KC, Rick P, Kyung KM, et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19(6):1170–9.
M M, J H, C LH, Y TW, Y C, W PS, et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: a randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology. 2019;8(8):1615817.
Isabella L, Wiebke W, Raphael M, Christoph R, Frank T, Linda H. In Situ Vaccination as a Strategy to Modulate the Immune Microenvironment of Hepatocellular Carcinoma. Front Immunol. 2021;12: 650486.
Zheng C, Zheng L, Yoo J-K, Guo H, Zhang Y, Guo X, et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169(7):1342-56.e16.
Bian J, Lin JZ, Long JY, Yang X, Yang XB, Lu X, et al. T lymphocytes in hepatocellular carcinoma immune microenvironment: insights into human immunology and immunotherapy. Am J Cancer Res. 2020;10(12):4585–606.
Faroogh M, Roza M, A. SV, Lakshmi T, Valerievich YA, Markov A, et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. 2021;12(1) 81.
Liu X, Wen JY, Yi HL, Hou XR, Yin Y, Ye GF, et al. Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release. Ther Adv Med Oncol. 2020;12:1758835920910347.
Li D, Li N, Zhang Y-F, Fu H, Feng M, Schneider D, et al. Persistent Polyfunctional Chimeric Antigen Receptor T Cells That Target Glypican 3 Eliminate Orthotopic Hepatocellular Carcinomas in Mice. Gastroenterology. 2020;158(8):2250-65.e20.
Xiaoyu L, Fang G, Longwei J, Meng J, Lei A, Ming L, et al. 32A9, a novel human antibody for designing an immunotoxin and CAR-T cells against glypican-3 in hepatocellular carcinoma. J Transl Med. 2020;18(1):295.
Luan S, Fang G, Zhanhui G, Lei A, Na L, Sujuan M, et al. Shed antigen-induced blocking effect on CAR-T cells targeting Glypican-3 in Hepatocellular Carcinoma. J Immunother Cancer. 2021;9(4): e001875.
Mengke N, Ming Y, Ning L, Kongju W, Kongming W. Advances of Targeted Therapy for Hepatocellular Carcinoma. Front Oncol. 2021;11: 719896.
Li KS, Qian SY, Huang MM, Chen MJ, Peng L, Liu JW, et al. Development of GPC3 and EGFR-dual-targeting chimeric antigen receptor-T cells for adoptive T cell therapy. American Journal of Translational Research. 2021;13(1):156–67.
Shunsuke K, Hidenori O, Hitoshi T, Jun H, Chigusa M, Masafumi I, et al. Clinical impact of c-Met expression and its gene amplification in hepatocellular carcinoma. Int J Clin Oncol. 2013;18(2):207–13.
Eh Y, Eh E, Mm M, Am R, Joydeep B, Susan E, et al. Norstictic Acid Inhibits Breast Cancer Cell Proliferation, Migration, Invasion, and In Vivo Invasive Growth Through Targeting C-Met. Phytotherapy research : PTR. 2016;30(4):557–66.
Katie H, Neil C, Nicola J, Rebecca L. The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front Immunol. 2020;11: 568931.
Wei J, Tao L, Jiaojiao G, Jingjing W, Lizhou J, Xiao S, et al. Bispecific c-Met/PD-L1 CAR-T cells have enhanced therapeutic effects on hepatocellular carcinoma. Front Oncol. 2021;11:546586.
Guo X, Jiang H, Shi B, Zhou M, Zhang H, Shi Z, et al. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol. 2018;9:1118.
Wu X, Luo H, Shi B, Di S, Sun R, Su J, et al. Combined antitumor effects of sorafenib and GPC3-CAR T Cells in mouse models of hepatocellular carcinoma. Mol Ther. 2019;27(8):1483–94.
Liu L, Bi E, Ma X, Xiong W, Qian J, Ye L, et al. Enhanced CAR-T activity against established tumors by polarizing human T cells to secrete interleukin-9. Nat Commun. 2020;11(1):5902.
Hanren D, Chuan T, Daiwei S, Meixia C, Yelei G, Deyun C, et al. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: a single-arm, open-label, phase II trial. Oncoimmunology. 2020;9(1):1846926.
Donghua S, Yaoping S, O KA, Xingxing Q, Yuan Z, Jiachang C, et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26(15):3895.
Konstantinos D, Hamza A, Nikolaos P. Cellular based treatment modalities for unresectable hepatocellular carcinoma. World J Clin Oncol. 2021;12(5):290–308.
Funding
This study was supported by National Natural Science Foundation of China (No. 81902914); Jiangsu Provincial Medical Youth Talent (No. QNRC2016043); and the Key Medical Science and Technology Development Project of Nanjing (No. ZKX16032.
Author information
Authors and Affiliations
Contributions
Mingzhen Zhou and Jie Shen composed the manuscript and provided figures. Baorui Liu reviewed and edited the manuscript. All authors interpreted the data, critically revised the manuscript for important intellectual contents and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, M., Liu, B. & Shen, J. Immunotherapy for hepatocellular carcinoma. Clin Exp Med 23, 569–577 (2023). https://doi.org/10.1007/s10238-022-00874-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00874-5