Abstract
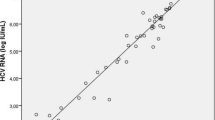
Value of hepatitis C virus (HCV) core antigen (cAg) test has been controversy in patients with low HCV loads for its lower sensitivity. We assessed correlation between HCV-cAg and HCV RNA in serum samples with low viral loads and analyzed the performance of HCV-cAg assay in determining diagnosis and treatment outcomes in chronic hepatitis C patients. Both HCV RNA and HCV-cAg were detected for 2298 serum samples. Correlation analysis was performed between the two tests. Receiver operating characteristics (ROC) curve was used to assess value of HCV-cAg test in determining diagnosis and response outcomes at the different HCV RNA thresholds. The two tests were correlated very well, and moreover, correlation in the low viral load group was higher than that in the high viral load group (r value: 0.901 and 0.517). Positive agreement of HCV-cAg ≥ 3 fmol/L was as high as 97.0% for HCV RNA ≥ 1000 IU/mL, and its negative agreement for HCV RNA < 15 IU/mL was up to 98.9% in all samples. Area under ROCs ranged from 0.939 to 0.992, regardless of HCV RNA thresholds. When lower limit of detection of HCV RNA was 15, 100 or 1000 IU/mL, positive predictive value of HCV-cAg was 96.8%, 98.8% or 92.4%, and its negative predictive value was 87.0%, 89.9% or 98.3%, respectively, on the basis of different cutoff values. High-sensitivity HCV-cAg detection may likely replace HCV RNA to confirm the existence of HCV and to guide the treatment of chronic HCV infection.




Similar content being viewed by others
References
World Health Organization. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en/. Accessed Mar 6 2019.
Jiang HJ, Wang XX, Luo BF, et al. Direct antiviral agents upregulate natural killer cell potential activity in chronic hepatitis C patients. Clin Exp Med. 2019;19:299–308.
Wróblewska A, Lorenc B, Cheba M, Bielawski KP, Sikorska K. Neutrocyte-to-lymphocyte ratio predicts the presence of a replicative hepatitis C virus strand after therapy with direct-acting antivirals. Clin Exp Med. 2019;19:401–6.
World Health Organization. Hepatitis C. https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c. Accessed Mar 6 2019.
Nguyen LT, Gray E, O’Leary A, Carr M, De Gascun CF. Irish hepatitis C outcomes research network. The role of hepatitis C virus core antigen testing in the era of direct acting antiviral therapies: what we can learn from the protease inhibitors. PLoS One. 2016;11:e0163900.
Jülicher P, Galli C. Identifying cost-effective screening algorithms for active hepatitis C virus infections in a high prevalence setting. J Med Econ. 2018;21:1–10.
Takahashi K, Okamoto H, Kishimoto S, et al. Demonstration of a hepatitis C virus-specific antigen predicted from the putative core gene in the circulation of infected hosts. J Gen Virol. 1992;73:667–72.
Freiman JM, Tran TM, Schumacher SG, et al. Hepatitis C core antigen testing for diagnosis of hepatitis C virus infection: a systematic review and meta-analysis. Ann Intern Med. 2016;165:345–55.
Chang C, Hung CH, Wang JH, Lu SN. Hepatitis C core antigen highly correlated to HCV RNA. Kaohsiung J Med Sci. 2018;34:684–8.
Cetiner S, Duran AC, Kibar F, Yaman A. Performance comparison of new generation HCV core antigen test versus HCV RNA test in management of hepatitis C virus infection. Transfus Apher Sci. 2017;56:362–6.
Lamoury FMJ, Hajarizadeh B, Soker A, et al. Evaluation of a hepatitis C virus core antigen assay in plasma and dried blood spot samples. J Mol Diagn. 2018;20:621–7.
Feng B, Yang RF, Zhang HY, et al. Early predictive efficacy of core antigen on antiviral outcomes in genotype 1 hepatitis C virus infected patients. Braz J Infect Dis. 2015;19:390–8.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420.
Ghanbari K, Roushani M, Azadbakht A. Ultra-sensitive aptasensor based on a GQD nanocomposite for detection of hepatitis C virus core antigen. Anal Biochem. 2017;534:64–9.
Pleshakova TO, Kaysheva AL, Bayzyanova JM, et al. The detection of hepatitis C virus core antigen using afm chips with immobolized aptamers. J Virol Methods. 2018;251:99–105.
Pleshakova TO, Kaysheva AL, Shumov ID, et al. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromach Basel. 2019;10:129.
Murdaca G, Contini P, Cagnati P, et al. Behavior of soluble HLA-A, -B, -C and HLA-G molecules in patients with chronic hepatitis C virus infection undergoing pegylated interferon-α and ribavirin treatment: potential role as markers of response to antiviral therapy. Clin Exp Med. 2017;17:93–100.
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511.
AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54.
Terrault NA, Pawlotsky JM, McHutchison J, et al. Clinical utility of viral load measurements in individuals with chronic hepatitis C infection on antiviral therapy. J Viral Hepat. 2005;12:465–72.
Ticehurst JR, Hamzeh FM, Thomas DL. Factors affecting serum concentrations of hepatitis C virus (HCV) RNA in HCV genotype 1-infected patients with chronic hepatitis. J Clin Microbiol. 2007;45:2426–33.
Arboledas JCA, Guerrero IP, Rodríguez MJB, et al. Hepatitis C virus core antigen in the management of patients treated with new direct-acting antivirals. Diagn Microbiol Infect Dis. 2017;89:29–34.
Kuo YH, Chang KC, Wang JH, et al. Is hepatitis C virus core antigen an adequate marker for community screening? J Clin Microbiol. 2012;50:1989–93.
Medici MC, Furlini G, Rodella A, et al. Hepatitis C virus core antigen: analytical performances, correlation with viremia and potential applications of a quantitative, automated immunoassay. J Clin Virol. 2011;51:264–9.
Miedouge M, Saune K, Kamar N, Rieu M, Rostaing L, Izopet J. Analytical evaluation of HCV core antigen and interest for HCV screening in haemodialysis patients. J Clin Virol. 2010;48:18–21.
Acknowledgements
The assistance of Abbott Diagnostics in the provision of the HCV core antigen kits for this evaluation.
Funding
Supported by Grants from National S&T Major Project for Infectious Diseases Control (2012ZX10002-003), National Major S&T Special Project for “Significant New Drugs Development” (2012ZX09303-019) and Beijing Medical & Health Technology Promotion Project (2018-TG-19).
Author information
Authors and Affiliations
Contributions
Feng B contributed to design, data acquisition and statistical analysis and drafted the manuscript; Yang RF and Jiang HJ contributed to data acquisition and statistical analysis; Xie YD and Zhang HY participated in study design and data analysis; Jin Q and Cong X participated in study planning and statistical analysis; Wei L contributed to funding, design and critical revision of the manuscript; and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed according to the guidelines of the International Conference on Harmonization for Good Clinical Practice and the ethical guidelines of the 1975 Declaration of Helsinki. The study protocol was approved by the ethical committees of Peking University People’s Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, B., Yang, RF., Jiang, HJ. et al. Correlation analysis of hepatitis C virus core antigen and low viral loads: Can core antigen replace nucleic acid test?. Clin Exp Med 20, 131–141 (2020). https://doi.org/10.1007/s10238-019-00588-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-019-00588-1