Abstract
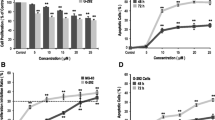
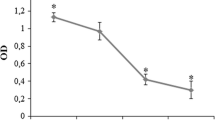
The purposes of this study were to investigate the effects of erythropoietin (EPO) on the proliferation and function of human osteoblast cells (hFOB 1.19) cultured in vitro and to explore the underlying molecular mechanisms to provide a theoretical foundation for clinical applications of EPO in oral implant and restoration therapies. Cultured hFOB 1.19 cells were treated with high and low doses of EPO. Changes in cell viability after 24 and 48 h of treatment were evaluated with the Mosmann tetrazolium assay. Changes in cell proliferation after 48 h of EPO treatment were measured by bromodeoxyuridine (BrdU) labeling, and changes in alkaline phosphatase (ALP) activity were determined by a specific assay. The effects of EPO on osteocalcin secretion were determined with the enzyme-linked immunosorbent assay, and changes in the protein expression of osteoprotegerin (OPG), osteopontin (OPN) and receptor activator of NF-κB ligand (RANKL) were assayed by western blot. The effects of EPO treatment on the levels of the EPO receptor (EPOR), phosphorylated Jak2 (P-Jak2) and phosphorylated Stat3 (P-Stat3) in hFOB 1.19 cells were evaluated in conjunction with a Jak2/Stat3 inhibitor. After 24 h of EPO treatment, hFOB 1.19 cells showed increased cell viability compared with the blank control group (p < 0.05). After 48 h, cell viability and growth were further improved relative to controls, with a significant increase observed for viability (p < 0.05). A significant increase in the proportion of BrdU-labeled proliferating cells was observed in the high-dose EPO group (p < 0.05), and EPO-treated cells also showed enhanced ALP activity (p < 0.05). There were no statistically significant differences in osteocalcin secretion between groups after 48 h of EPO treatment (p > 0.05); however, increased secretion was observed in EPO-treated cells after 96 h of treatment (p < 0.05). EPO treatment significantly promoted OPG and OPN expression (p < 0.05) while significantly inhibiting RANKL expression (p < 0.01). EPO treatment also significantly upregulated the levels of EPOR, P-Jak2 and P-Stat3 in hFOB 1.19 cells (p < 0.01); these effects were abrogated by co-treatment with a Jak2/Stat3 inhibitor (AG490) (p < 0.01). EPO significantly stimulated osteoblast proliferation and differentiation. The underlying molecular mechanism is associated with the ability of EPO to promote ALP activity, osteocalcin secretion and OPG and OPN expression and to inhibit RANKL expression in osteoblasts. This mechanism appears to be mediated by the Jak2/Stat3 pathway.






Similar content being viewed by others
References
Seong WJ, Kim HC, Jeong S, Deveau DL, Aparicio C, Li Y, Hodges JS (2011) Ex vivo mechanical properties of dental implant bone cement used to rescue initially unstable dental implants: a rabbit study. Int J Oral Maxillofac Implants 26:826–836
Al-Omiri MK, Hammad OA, Lynch E, Lamey PJ, Clifford TJ (2011) Impacts of implant treatment on daily living. Int J Oral Maxillofac Implants 26:877–886
Carpentier B, Layrolle P, Legallais C (2011) Bioreactors for bone tissue engineering. Int J Artif Organs 34:259–270
Bajek A, Olkowska J, Drewa T (2011) Mesenchymal stem cells as a therapeutic tool in tissue and organ regeneration. Postepy Hig Med Dosw (Online) 65:124–132
Li MAmizuka N (2006) Histopathological observations on osteolytic bone metastasis. Clin Calcium 16:591–597
Gundberg CM (2000) Biochemical markers of bone formation. Clin Lab Med 20:489–501
El Haj AJ, Cartmell SH (2010) Bioreactors for bone tissue engineering. Proc Inst Mech Eng H 224:1523–1532
Dong S, Yang B, Guo H, Kang F (2012) MicroRNAs regulate osteogenesis and chondrogenesis. Biochem Biophys Res Commun 418:587–591
Chen G, Deng C, Li YP (2012) TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8:272–288
Rathod DB, Salahudeen AK (2011) Nonerythropoietic properties of erythropoietin: implication for tissue protection. J Investig Med 59:1083–1085
Chateauvieux S, Grigorakaki C, Morceau F, Dicato M, Diederich M (2011) Erythropoietin, erythropoiesis and beyond. Biochem Pharmacol 82:1291–1303
Sorg H, Harder Y, Krueger C, Reimers K, Vogt PM (2012) The nonhematopoietic effects of erythropoietin in skin regeneration and repair: from basic research to clinical use. Med Res Rev. doi:10.1002/med.21259
McGee SJ, Havens AM, Shiozawa Y, Jung Y, Taichman RS (2012) Effects of erythropoietin on the bone microenvironment. Growth Factors 30:22–28
Moore E, Bellomo R (2011) Erythropoietin (EPO) in acute kidney injury. Ann Intensive Care 1:3
Holstein JH, Orth M, Scheuer C, Tami A, Becker SC, Garcia P, Histing T, Morsdorf P, Klein M, Pohlemann T, Menger MD (2011) Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone 49:1037–1045
Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, Wang J, Krebsbach PH, Taichman RS (2012) Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem 113:220–228
Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Lee CH, Sud S, Pienta KJ, Krebsbach PH, Taichman RS (2010) Erythropoietin couples hematopoiesis with bone formation. PLoS ONE 5:e10853
Chiba T, Yamada M, Aiso S (2009) Targeting the JAK2/STAT3 axis in Alzheimer’s disease. Expert Opin Ther Targets 13:1155–1167
Duan W, Yang Y, Yan J, Yu S, Liu J, Zhou J, Zhang J, Jin Z, Yi D (2012) The effects of curcumin post-treatment against myocardial ischemia and reperfusion by activation of the JAK2/STAT3 signaling pathway. Basic Res Cardiol 107:1–12
Boykin C, Zhang G, Chen YH, Zhang RW, Fan XE, Yang WM, Lu Q (2012) Cucurbitacin IIa: a novel class of anti-cancer drug inducing non-reversible actin aggregation and inhibiting survivin independent of JAK2/STAT3 phosphorylation. Br J Cancer 106:1248
Annunziata M, Oliva A, Basile MA, Giordano M, Mazzola N, Rizzo A, Lanza A, Guida L (2011) The effects of titanium nitride-coating on the topographic and biological features of TPS implant surfaces. J Dent 39:720–728
Anchieta RB, Rocha EP, Watanabe MU, de Almeida EO, Freitas-Junior AC, Martini AP, Barioni SR (2011) Recovering the function and esthetics of fractured teeth using several restorative cosmetic approaches. Three clinical cases. Dent Traumatol 28:166–172
Orsini G, Stacchi C, Visintini E, Di Iorio D, Putignano A, Breschi L, Di Lenarda R (2011) Clinical and histologic evaluation of fresh frozen human bone grafts for horizontal reconstruction of maxillary alveolar ridges. Int J Periodontics Restorative Dent 31:535–544
Stein SH, Tipton DA (2011) Vitamin D and its impact on oral health–an update. J Tenn Dent Assoc 91:30–33; quiz 34–35
Horvai AE, Boyce BF (2011) Metabolic bone diseases. Semin Diagn Pathol 28:13–25
Iwamoto J, Sato Y, Takeda T, Matsumoto H (2011) Bone quality and vitamin K2 in type 2 diabetes: review of preclinical and clinical studies. Nutr Rev 69:162–167
Reichel C (2011) Recent developments in doping testing for erythropoietin. Anal Bioanal Chem 401:463–481
Holstein JH, Menger MD, Scheuer C, Meier C, Culemann U, Wirbel RJ, Garcia P, Pohlemann T (2007) Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci 80:893–900
Pivodova V, Frankova J, Ulrichova J (2011) Osteoblast and gingival fibroblast markers in dental implant studies. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155:109–116
Orimo H (2011) The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nihon Med Sch 77:4–12
Villafan-Bernal JR, Sanchez-Enriquez S, Munoz-Valle JF (2011) Molecular modulation of osteocalcin and its relevance in diabetes (Review). Int J Mol Med 28:283–293
Marolt D, Cozin M, Vunjak-Novakovic G, Cremers S, Landesberg R (2011) Effects of pamidronate on human alveolar osteoblasts in vitro. J Oral Maxillofac Surg 70:1081–1092
Reyes-Garcia R, Rozas-Moreno P, Munoz-Torres M (2011) Cardiovascular disease and bone metabolism. Endocrinol Nutr 58:353–359
Cho HJ, Kim HS (2009) Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep 11:206–213
Saidenberg Kermanac’h N, Bessis N, Cohen-Solal M, De Vernejoul MC, Boissier MC (2002) Osteoprotegerin and inflammation. Eur Cytokine Netw 13:144–153
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Jelkmann W (2007) Control of erythropoietin gene expression and its use in medicine. Methods Enzymol 435:179–197
Aapro M, Jelkmann W, Constantinescu SN, Leyland-Jones B (2012) Effects of erythropoietin receptors and erythropoiesis-stimulating agents on disease progression in cancer. Br J Cancer 106:1249–1258
Acknowledgments
This work was supported by Administration of Traditional Chinese Medicine of Guangdong Province (20111264) and Research Foundation of Science & Technology Bureau of Liwan District of Guangzhou city (20111213053). The authors are grateful to Dr Aaron L for editing the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, L., Luo, T., Fang, Y. et al. Effects of erythropoietin on osteoblast proliferation and function. Clin Exp Med 14, 69–76 (2014). https://doi.org/10.1007/s10238-012-0220-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-012-0220-7