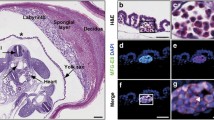
Abstract
In an initial period of vertebrate phylogeny (bone marrow-less vertebrates), lymphohaematopoiesis takes place in numerous organs containing a suitable microenvironment. Among other organs (i.e., gonads, kidney and spleen), the liver is apparently the most appropriate site for homing and differentiation of haematopoietic cell precursors. Interaction between haematopoietic cells and stromal cells is important for regulation of haematopoiesis. Numerous soluble and membrane-bound factors directly regulating haematopoiesis have been documented, but little is known about the effect of the foetal hepatic epithelial-to-mesenchymal transition (EMT) stromal cells’ activity and their product-fibronectin, on foetal hepatic haematopoiesis. The binding of late-stage erythroid cells to FN has been well characterised and is believed to be critical for the terminal stages of erythroid differentiation. The intention of this article is to provide a quantitative overview of FN, produced by hepatic EMT stromal cells, in foetal hepatic haematopoiesis during the first and second trimester of development. Paraffin-embedded specimens from the liver of 30 human embryos in the first and second trimesters of gestation were investigated by conventional histology and immunohistology for the presence of FN and specific haematopoietic cell types. The staining intensity, and localisation of FN and haematopoietic markers in sequential sections were examined. Furthermore, double immunohistochemical staining was performed to assess simultaneous detection of FN and haematopoietic markers. FN was expressed in the EMT stromal cells of the hepatic portal triads more strongly during the second trimester than the first. Furthermore, an intense immunostaining for haematopoietic lineages, and especially for erythropoiesis, was observed in the second trimester compared to the first. The results of the double immunostaining disclosed an intimate co-expression of the FN and CD haematopoietic markers. Foetal hepatic EMT stromal cells provide a unique microenvironment that supports the emergence, expansion and maintenance of human foetal haematopoietic development during the mid-gestational stage. FN produced by the EMT stromal cells follows a time course parallel to that of haematopoiesis. We suggest that in foetal liver, phenotypic modifications of EMT stromal cells expressing FN concerning the cell adhesion capacity of the protein are associated with proliferation and differentiation of specific haematopoietic cell lineages during the second trimester of gestation, probably reflecting the increasing demand of the growing foetus for mature erythroid and myeloid cells.
Similar content being viewed by others
References
Charbord P, Tavian M, Coulombel L et al (1995) Early ontogeny of the human hematopoietic system. C R Seances Soc Biol Fil 189:601–609
Tavian M, Coulombel L, Loton D et al (1996) Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood 87:67–72
Potonic AJ, Brakebush C, Fassler R (2000) Fetal and adult hematopoietic stem cells require beta 1 integrin function for colonizing fetal liver, spleen and bone marrow. Immunity 12:653–663
Chagraoui J, Lepage-Noll A, Anjo A et al (2003) Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood 101:2973–2982
Viebahn C (1995) Epithelio-mesenchymal transformation during formation of the mesoderm in the mammalian embryo. Acta Anat 154:79–97
Davies JA (1996) Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat 156:187–201
Pagan R, Liobera M, Vilaro S (1995) Epithelial-mesenchymal transition in cultured neonatal hepatocytes. Hepatology 21:820–831
Kiassov AP, Van Eyken P, van Pelt JF et al (1995) Desmin expressing nonhematopoietic liver cells during rat liver development: an immunohistochemical and morphometric study. Differentiation 59:253–258
Martin MA, Bhatia M (2005) Analysis of the human liver hematopoietic microenvironment. Stem Cells Dev 14:493–504
Charbord P, Oostendorp R, Pang W et al (2002) Comparative study of stromal cell lines derived from embryonic, fetal, and postnatal mouse blood-forming tissues. Exp Hematol 30:1202–1210
Oostendorp RA, Medvinsky AJ, Kusadasi N et al (2002) Embryonal subregion-derived stromal cell lines from novel temperature-sensitive SV40 T antigen transgenic mice support hematopoiesis. J Cell Sci 115:2099–2108
Oostendorp RA, Robin C, Steinhoff C et al (2005) Long-term maintenance of hematopoietic stem cells does not require contact with embryo-derived stromal cells in cocultures. Stem Cells 23:842–851
Zhang H, Miao Z, He Z et al (2005) The existence of epithelial-to-mesenchymal cells with the ability to support hematopoiesis in human fetal liver. Cell Biol Int 29:213–219
Yokota T, Oritani K, Mitsui H et al (1998) Growth-supporting activities of fibronectin on hematopoietic stem/progenitor cells in vitro and in vivo: structural requirement for fibronectin activities of CS1 and cell-binding domains. Blood 91:3263–3272
Goltry KL, Patel VP (1997) Specific domains of fibronectin mediate adhesion and migration of early murine erythroid progenitors. Blood 90:138–147
Miyamoto S, Katz BZ, Lafrenie RM, Yamada KM (1998) Fibronectin and integrins in cell adhesion, signaling, and morphogenesis. Ann N Y Acad Sci 857:119–129
Vogel W, Berndt A, Muller A et al (2003) Differential in vivo and in vitro expression of ED-B+ fibronectin in adult human hemopoiesis. Int J Mol Med 12:831–837
Gotoh A, Ritchie A, Takahira H, Broxmeyer HE (1997) Thrombopoietin and erythropoietin activate inside-out signaling of integrin and enhance adhesion to immobilized fibronectin in human growth-factor-dependent hematopoietic cells. Ann Hematol 75:207–213
Goltry KL, Patel VP (1997) Specific domains of fibronectin mediate adhesion and migration of early murine erythroid progenitors. Blood 90:138–147
Hassan HT, Sadovinkova E, Drize NJ et al (1997) Fibronectin increases both non-adherent cells and CFU-GM while collagen increases adherent cells in human normal long-term bone marrow cultures. Haematologia 28:77–84
Hackney JA, Charbord P, Brunk BP et al (2002) A molecular profile of a hematopoietic stem cell niche. Proc Natl Acad Sci U S A 99:13061–13066
Hackney JA, Moore KA (2005) A functional genomics approach to hematopoietic stem cell regulation. Methods Mol Med 105:432–452
Charbord P, Moore K (2005) Gene expression in stem cell-supporting stromal cell lines. Ann N Y Acad Sci 1044:159–167
Watt F, Hogan B (2000) Out of Eden: stem cells and their niches. Science 287:1427–1430
Timens W, Kamps WA (1997) Hemopoiesis in human fetal and embryonic liver. Microsc Res Tech 39:387–397
Norton AJ, Jordan S, Yeomans P (1994) Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol 173:371–379
Cordell JL, Falini B, Erber WN et al (1984) Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 32:219–229
Gilles JM, Divon MY, Bentolila E et al (1997) Immunophenotypic characterization of human fetal liver hematopoietic stem cells during the midtrimester of gestation. Am J Obstet Gynecol 177:619–625
Pahal GS, Jauniaux E, Kinnon C et al (2000) Normal development of human fetal hematopoiesis between eight and seventeen weeks’s gestation. Am J Obstet Gynecol 183:1029–1034
Weinstein R, Riordan MA, Wenc K et al (1989) Dual role of fibronectin in hematopoietic differentiation. Blood 73:111–116
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lambropoulou, M., Tamiolakis, D., Venizelos, I. et al. Induction of hepatic haematopoiesis with fibronectin expression by EMT stromal cells during the second trimester of development. Clin. Exper.Med. 7, 115–121 (2007). https://doi.org/10.1007/s10238-007-0132-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-007-0132-0