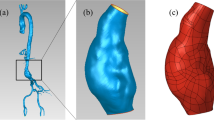
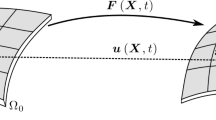Abstract
In its permanent quest of mechanobiological homeostasis, our vasculature significantly adapts across multiple length and timescales in various physiological and pathological conditions. Computational modeling of vascular growth and remodeling (G&R) has significantly improved our insights into the mechanobiological processes of diseases such as hypertension or aneurysms. However, patient-specific computational modeling of ascending thoracic aortic aneurysm (ATAA) evolution, based on finite element models (FEM), remains a challenging scientific problem with rare contributions, despite the major significance of this topic of research. Challenges are related to complex boundary conditions and geometries combined with layer-specific G&R responses. To address these challenges, in the current paper, we employed the constrained mixture model (CMM) to model the arterial wall as a mixture of different constituents such as elastin, collagen fiber families and smooth muscle cells. Implemented in Abaqus as a UMAT, this first patient-specific CMM-based FEM of G&R in human ATAA was first validated for canonical problems such as single-layer thick-wall cylindrical and bilayer thick-wall toric arterial geometries. Then it was used to predict ATAA evolution for a patient-specific aortic geometry, showing that the typical shape of an ATAA can be simply produced by elastin proteolysis localized in regions of deranged hemodymanics. The results indicate a transfer of stress to the adventitia by elastin loss and continuous adaptation of the stress distribution due to change in ATAA shape. Moreover, stress redistribution leads to collagen deposition where the maximum elastin mass is lost, which in turn leads to stiffening of the arterial wall. As future work, the predictions of this G&R framework will be validated on datasets of patient-specific ATAA geometries followed up over a significant number of years.











Similar content being viewed by others
Abbreviations
- \(\mathbf{a}_0^k\) :
-
The unit vector pointing direction of the kth fiber
- \(\mathbf{C}^i_{{\text {el}}}\) :
-
Elastic right Cauchy–Green deformation tensor of the ith constituent
- \(\overline{\mathbf{C}}^i_{{\text {el}}}\) :
-
Modified elastic right Cauchy–Green deformation tensor of the ith constituent
- \(D_{\text {max}}\) :
-
Maximum damage of elastin
- \(\dot{D}^i_{\mathrm{g}}\) :
-
Generic rate function of ith constituent
- \(\mathbf{F}\) :
-
Total deformation gradient of the mixture
- \(\mathbf{F}^i_{\text {tot}}\) :
-
Total deformation gradient of the ith constituent
- \(\mathbf{F}^i_{\text {el}}\) :
-
Elastic deformation gradient of the ith constituent
- \(\mathbf{F}^i_{\text {gr}}\) :
-
Total inelastic (G&R) deformation gradient of the ith constituent
- \(\mathbf{F}^i_{\text {g}}\) :
-
Deformation gradient of the ith constituent due to growth
- \(\mathbf{F}^i_{\text {r}}\) :
-
Deformation gradient of the ith constituent due to remodeling
- \(\mathbf{G}^i_{\mathrm{h}}\) :
-
Deposition stretch tensor of the ith constituent
- J :
-
Jacobian of the mixture
- \(\overline{I}_1^i\) :
-
First invariant of the right Cauchy–Green deformation tensor for the ith constituent
- \({I}_4^i\) :
-
Fourth invariant of the right Cauchy–Green deformation tensor for the ith constituent
- \(k^{{\text {c}}_j}_\sigma\) :
-
Gain or growth parameter of collagen fiber families
- \(k^k_1\) :
-
Fung-type material coefficient the kth constituent
- \(k^k_2\) :
-
Fung-type material coefficient the kth constituent
- \(L_{\text {dam}}\) :
-
Spatial damage spread parameter of elastin
- \(\mathbf{S}\) :
-
Second Piola–Kirchhoff stress
- \(T^i\) :
-
Average turnover time of the ith constituent
- \(t_{\text {dam}}\) :
-
Temporal damage spread parameter of elastin
- W :
-
The specific strain energy density function of the mixture
- \({W}^i\) :
-
Strain energy of the ith individual constituents
- \(\mathbf{X}\) :
-
Material point in a reference configuration
- \(\mathbf{x}\) :
-
Material point in a deformed or current configuration
- \(\alpha ^{c_{j}}\) :
-
Each direction of collagen fiber families
- \(\mu ^{{\text {e}}}\) :
-
Neo-Hookean material coefficient of elastin
- \(\kappa\) :
-
Bulk modulus of elastin
- \(\sigma ^i\) :
-
Current stress of extant ith constituent
- \(\sigma ^{{\text {c}}_j}_{\mathrm{h}}\) :
-
Average stress of ith constituent at homeostasis
- \(\lambda ^{{\text {e}}}_z\) :
-
Axial elastin deposition stretch value
- \(\lambda ^{{\text {e}}}_\theta\) :
-
Circumferential elastin deposition stretch value
- \(\lambda ^k\) :
-
Deposition stretch value of kth constituent in fiber direction
- \({\varvec{\Omega }}_0\) :
-
Reference configuration
- \({\varvec{\Omega }}(t)\) :
-
Deformed or current configuration
- \(\varrho ^i_0\) :
-
Mass densities of the ith constituent before G&R
- \(\varrho ^i_t\) :
-
Mass densities of the ith constituent at time t
- \(\dot{\varrho }^{{{\text {e}}}}(t)\) :
-
Rate of mass degradation of the elastin
- \(\dot{\varrho }^{{\text {c}}_j}_{\text {adv}}(t)\) :
-
Rate of mass degradation or deposition in the adventitia for collagen fibers
- \(\dot{\varrho }^{{\text {c}}_j}_{\text {med}}(t)\) :
-
Rate of mass degradation or deposition in the media for collagen fibers
References
Alford PW, Taber LA (2008) Computational study of growth and remodelling in the aortic arch. Comput Methods Biomech Biomed Eng 11(5):525–38
Baek S, Rajagopal KR, Humphrey JD (2006) A theoretical model of enlarging intracranial fusiform aneurysms. J Biomech Eng 128(1):142–9
Baek S, Valentín A, Humphrey JD (2007) Biochemomechanics of cerebral vasospasm and its resolution: II. Constitutive relations and model simulations. Ann Biomed Eng 35:1498-509
Bellini C, Ferruzzi J, Roccabianca S, Di Martino ES, Humphrey JD (2014) A microstructurally motivated model of arterial wall mechanics with mechanobiological implications. Ann Biomed Eng 42(3):488–502
Bersi MR, Bellini C, Di Achille P, Humphrey JD, Genovese K, Avril S (2016) Novel methodology for characterizing regional variations in the material properties of murine aortas. J Biomech Eng 138(7):11. https://doi.org/10.1115/1.4033674
Braeu FA, Seitz A, Aydin RC, Cyron CJ (2017) Homogenized constrained mixture models for anisotropic volumetric growth and remodeling. Biomech Model Mechanobiol 16(3):889–906
Cardamone L, Humphrey JD (2012) Arterial growth and remodelling is driven by hemodynamics. In: Ambrosi D, Quarteroni A, Rozza G (eds) Modeling of physiological flows, MS&A—modeling, simulation and applications. Springer, Milan
Cardamone L, Valentin A, Eberth JF, Humphrey JD (2009) Origin of axial prestretch and residual stress in arteries. Biomech Model Mechanobiol 8:431–46
Cardamone L, Valentín A, Eberth JF, Humphrey JD (2010) Modelling carotid artery adaptations to dynamic alterations in pressure and flow over the cardiac cycle. Math Med Biol 27(4):343–71
Comellas E, Gasser TC, Bellomo FJ, Oller S (2016) A homeostatic-driven turnover remodelling constitutive model for healing in soft tissues. J R Soc Interface 13(116):12. https://doi.org/10.1098/rsif.2015.1081
Condemi F, Campisi S, Viallon M, Troalen T, Xuexin G, Barker AJ, Markl M, Croisille P, Trabelsi O, Cavinato C, Duprey A, Avril S (2017) Fluid- and biomechanical analysis of ascending thoracic aorta aneurysm with concomitant aortic insufficiency. Ann Biomed Eng 45(12):2921–32
Cyron CJ, Humphrey JD (2016) Growth and remodeling of load-bearing biological soft tissues. Meccanica 52(3):645–64
Cyron CJ, Wilson JS, Humphrey JD (2014) Mechanobiological stability: a new paradigm to understand the enlargement of aneurysms? J R Soc Interface 11(100):20140680
Cyron CJ, Aydin RC, Humphrey JD (2016) A homogenized constrained mixture (and mechanical analog) model for growth and remodeling of soft tissue. Biomech Model Mechanobiol 15(6):1389–1403
Davis FM, Luo Y, Avril S, Duprey A, Lu J (2016) Local mechanical properties of human ascending thoracic aneurysms. J Mech Behav Biomed Mater 61:235–49
Di Achille P, Tellides G, Humphrey JD (2017) Hemodynamics-driven deposition of intraluminal thrombus in abdominal aortic aneurysms. Int J Numer Method Biomed Eng 33(5):e2828
Eriksson TSE (2014) Modelling volumetric growth in a thick-walled fibre reinforced artery. J Mech Phys Solids 73:134–150
Eriksson TSE, Watton PN, Luo XY, Ventikose Y (2014) Modelling volumetric growth in a thick-walled fibre reinforced artery. J Mech Phys Solids 73:134–50
Famaey N, Vastmans J, Fehervary H, Maes L, Vanderveken E, Rega F, Mousavi SJ, Avril S (2018) Numerical simulation of arterial remodeling in pulmonary autografts. Z Angew Math Mech 98:1–19
Farzaneh S, Paseta O, Gómez-Benito MJ (2015) Multi-scale finite element model of growth plate damage during the development of slipped capital femoral epiphysis. Biomech Model Mechanobiol 14(2):371–85
Farzaneh S, Trabelsi O, Avril S (2018) Inverse identification of local stiffness across ascending thoracic aortic aneurysms. Biomech Model Mechanobiol. https://doi.org/10.1007/s10237-018-1073-0
Farzaneh S, Trabelsi O, Chavent B, Avril S (2019) Identifying local arterial stiffness to assess the risk of rupture of ascending thoracic aortic aneurysms. Ann Biomed Eng 47(4):1038–50
Figueroa CA, Baek S, Taylor CA, Humphrey JD (2009) A computational framework for fluid–solid-growth modeling in cardiovascular simulations. Comput Methods Appl Mech Eng 198(45–46):3583–602
Gasser Ch, Grytsan A (2017) Biomechanical modeling the adaptation of soft biological tissue. Curr Opin Biomed Eng 1:71–77
Gee MW, Forster C, Wall WA (2010) A computational strategy for prestressing patient-specific biomechanical problems under finite deformation. Int J Numer Meth Biomed Eng 26:52–72
Grossman W (1980) Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med 69(4):576–84
Grytsan A, Watton PN, Holzapfel GA (2015) A thick-walled fluid–solid growth model of abdominal aortic aneurysm evolution: application to a patient-specific geometry. J Biomech Eng 137(3):031008
Guzzardi DG, Barker AJ, van Ooij P, Malaisrie SC, Puthumana JJ, Belke DD, Mewhort HE, Svystonyuk DA, Kang S, Verma S, Collins J, Carr J, Bonow RO, Markl M, Thomas JD, McCarthy PM, Fedak PW (2015) Valve-related hemodynamics mediate human bicuspid aortopathy: insights from wall shear stress mapping. J Am Coll Cardiol 66(8):892–900
Hibbit K, Sorensen (2011) Abaqus-theory manual, 6.11-3 edition
Holzapfel AG, Gasser CT, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61:1–48
Hope TA, Markl M, Wigström L, Alley MT, Miller DC, Herfkens RJ (2007) Comparison of flow patterns in ascending aortic aneurysms and volunteers using four-dimensional magnetic resonance velocity mapping. J Magn Reson Imaging 26(6):1471–9
Hosseini HS, Taber LA (2018) How mechanical forces shape the developing eye. Progr Biophys Mol Biol 137:1–12
Hosseini HS, Garcia KE, Taber LA (2017) A new hypothesis for foregut and heart tube formation based on differential growth and actomyosin contraction. Development 144(13):2381–2391
Humphrey JD (1995) Mechanics of arterial wall: review and directions. Crit Rev Biomed Eng 23(1–2):1–162
Humphrey JD (2008a) Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 52(2):195–200
Humphrey JD (2008b) Vascular adaptation and mechanical homeostatsis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys 50:53–78
Humphrey JD, Holzapfel GA (2012) Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J Biomech 45(5):805–84
Humphrey JD, Rajagopal KR (2002) A constrained mixture model for growth and remodeling of soft tissues. Math Models Methods Appl Sci 12(03):407–30
Humphrey JD, Schwartz MA, Tellides G, Milewicz DM (2015) Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ Res 116(8):1448–1461
Kars̆aj I, Sorić J, Humphrey JD (2010) A 3-D framework for arterial growth and remodeling in response to altered hemodynamics. Int J Eng Sci 48(11):1357–1372
Kassab GS (2008) Mechanical homeostasis of cardiovascular tissue. In: Artmann GM, Chien S (eds) Bioengineering in cell and tissue research. Springer, Berlin, pp 371–391
Latorre M, Humphrey JD (2018a) Critical roles of time-scales in soft tissue growth and remodeling. APL Bioeng 2:026108
Latorre M, Humphrey JD (2018b) Mechanobiological stability of biological soft tissues. J Mech Phys Solids 125:298–325
Lin WJ, Iafrati MD, Peattie RA, Dorfmann L (2017) Growth and remodeling with application to abdominal aortic aneurysms. J Eng Math 109(1):113–137
Maes L, Fehervary H, Vastmans J, Mousavi SJ, Avril S, Famaey N (2019) Constrained mixture modeling affects material parameter identification from planar biaxial tests. J Mech Behav Biomed Mater 95:124–35
Marsden AL, Feinstein JA (2015) Computational modeling and engineering in pediatric and congenital heart disease. Curr Opin Pediatr 27(5):587
Matsumoto T, Hayashi K (1996) Response of arterial wall to hypertension and residual stress. In: Hayashi K, Kamiya A, Ono K (eds) Biomechanics. Springer, Tokyo, pp 93–119
Mousavi SJ, Avril S (2017) Patient-specific stress analyses in the ascending thoracic aorta using a finite-element implementation of the constrained mixture theory. Biomech Model Mechanobiol. https://doi.org/10.1007/s10237-017-0918-2
Mousavi SJ, Farzaneh S, Avril S (2018) Computational predictions of damage propagation preceding dissection of ascending thoracic aortic aneurysms. Int J Numer Method Biomed Eng 34(4):e2944
Riveros F, Chandra S, Finol EA, Gasser TC, Rodriguez JF (2013) A pull-back algorithm to determine the unloaded vascular geometry in anisotropic hyperelastic aaa passive mechanics. Ann Biomed Eng 41(4):694–708
Rodriguez EK, Hoger A (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27(4):455–67
Rodriguez JF, Ruiz C, Doblaré M, Holzapfel G (2008) Mechanical stresses in abdominal aortic aneurysms: influence of diameter, asymmetry, and material anisotropy. ASME J Biomech 130(2):021023
Sheidaei A, Hunley SC, Zeinali-Davarani S, Raguin LG, Baek S (2011) Simulation of abdominal aortic aneurysm growth with updating hemodynamic loads using a realistic geometry. Med Eng Phys 33(1):80–88
Valentín A, Holzapfel GA (2012) Constrained mixture models as tools for testing competing hypotheses in arterial biomechanics: a brief survey. Mech Res Commun 42:126–33
Valentín A, Humphrey JD (2009a) Evaluation of fundamental hypotheses underlying constrained mixture models of arterial growth and remodelling. Philos Trans A Math Phys Eng Sci 367:3585–606
Valentín A, Humphrey JD (2009b) Parameter sensitivity study of a constrained mixture model of arterial growth and remodeling. J Biomech Eng 131:101006
Valentín A, Cardamone L, Baek S, Humphrey JD (2009) Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure. J R Soc Interface 6(32):293–306
Valentín A, Humphrey JD, Holzapfel GA (2011) A multi-layered computational model of coupled elastin degradation, vasoactive dysfunction, and collagenous stiffening in aortic aging. Ann Biomed Eng 39(7):2027–45
Valentín A, Humphrey JD, Holzapfel G (2013) A finite element-based constrained mixture implementation for arterial growth, remodeling, and adaptation: theory and numerical verification. Int J Numer Method Biomed Eng 29(8):822–49
Vorp DA, Geest JP Vande (2005) Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler Thromb Vasc Biol 25(8):1558–1566
Vorp DA, Lee PC, Wang DHJ, Makaroun MS, Nemoto EM, Ogawa S, Webster MW (2001) Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg 34(2):291–99
Watton PN, Hill NA (2009) Evolving mechanical properties of a model of abdominal aortic aneurysm. Biomech Model Mechanobiol 8(1):25–42
Watton PN, Hill NA, Heil M (2004) A mathematical model for the growth of the abdominal aortic aneurysm. Biomech Model Mechanobiol 3(2):98–113
Watton PN, Raberger NB, Holzapfel GA, Ventikos Y (2009) Coupling the hemodynamic environment to the evolution of cerebral aneurysms: computational framework and numerical examples. J Biomech Eng 131(10):10. https://doi.org/10.1115/1.3192141
Wilson JS, Baek S, Humphrey JD (2013) Parametric study of effects of collagen turnover on the natural history of abdominal aortic aneurysms. Proc R Soc A 469(2150):20120556
Zeinali-Davarani S, Baek S (2012) Medical image-based simulation of abdominal aortic aneurysm growth. Mech Res Commun 42:107–17
Zeinali-Davarani S, Sheidaei A, Baek S (2011) A finite element model of stress-mediated vascular adaptation: application to abdominal aortic aneurysms. Comput Methods Biomech Biomed Eng 14(9):803–17
Zhou X, Raghavan ML, Harbaugh RE, Lu J (2010) Specific wall stress analysis in cerebral aneurysms using inverse shell model. Ann Biomed Eng 38(2):478–89
Acknowledgements
The authors are grateful to the European Research Council for Grant ERC-2014-CoG BIOLOCHANICS. The authors would also like to thank Nele Famaey (KU Leuven, Belgium), Christian J. Cyron (TU Hamburg, Germany) and Fabian A. Braeu (TU München, Germany) for inspiring discussions related to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In the list of symbols, \(i \in \left\{ {{{\text {e}}}, {\text {c}}}_j, {{\text {m}}} \right\}\) and \(k \in \left\{ {\text {c}}_j, {{\text {m}}} \right\}\)
Rights and permissions
About this article
Cite this article
Mousavi, S.J., Farzaneh, S. & Avril, S. Patient-specific predictions of aneurysm growth and remodeling in the ascending thoracic aorta using the homogenized constrained mixture model. Biomech Model Mechanobiol 18, 1895–1913 (2019). https://doi.org/10.1007/s10237-019-01184-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-019-01184-8