Abstract
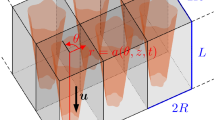
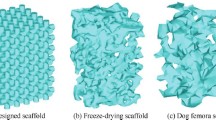
The control of the mechanical stimuli transmitted to the cells is critical for the design of functional scaffolds for tissue engineering. The objective of this study was to investigate the dynamics of the mechanical stimuli transmitted to the cells during tissue differentiation in an irregular morphology scaffold under compressive load and perfusion flow. A calcium phosphate-based glass porous scaffold was used. The solid phase and the fluid flow within the pores were modeled as linear elastic solid material and Newtonian fluid, respectively. In the fluid model, different levels of viscosity were used to simulate tissue differentiation. Compressive strain of 0.5% and fluid flow with constant inlet velocity of 10 μm/s or constant inlet pressure of 3 Pa were applied. Octahedral shear strain and fluid shear stress were used as mechano-regulatory stimuli. For constant inlet velocity, stimuli equivalent to bone were predicted in 80% of pore volume for the case of low tissue viscosity. For the cases of high viscosity, fluctuations between stimuli equivalent to tissue formation and cell death were predicted due to the increase in the fluid shear stress when tissue started to fill pores. When constant pressure was applied, stimuli equivalent to bone were predicted in 62% of pore volume when low tissue viscosity was used and 42% when high tissue viscosity was used. This study predicted critical variations of fluid shear stress when cells differentiated. If these variations are not controlled in vitro, they can impede the formation of new matured tissue.
Similar content being viewed by others
References
Byrne DP, Lacroix D, Planell JA, Kelly DJ, Prendergast PJ (2007) Simulation of tissue differentiation in a scaffold as a function of porosity Young’s modulus and dissolution rate: application of mechanobiological models in tissue engineering. Biomaterials 28: 5544–5554
Carter DR, Beaupré GS (2001) Skeletal function and form. Cambridge university press, Cambridge
Cartmell SH, Porter BD, Garcia AJ, Guldberg RE (2003) Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng 9: 1197–1203
Checa S, Prendergast PJ (2010) Effect of cell seeding and mechanical loading on vascularization and tissue formation inside a scaffold: a mechano-biological model using a lattice approach to simulate cell activity. J Biomech 43: 961–968
Cioffi M, Boschetti F, Raimondi MT, Dubini G (2006) Modeling evaluation of the fluid-dynamic microenvironment in tissue-engineered constructs: a micro-CT based model. Biotechnol Bioeng 93: 500–510
Glowacki J, Trepman E, Folkman J (1983) Cell shape and phenotypic expression in chondrocytes. Proc Soc Exp Biol Med 172: 93–98
Holtorf HL, Sheffield TL, Ambrose CG, Jansen AJ, Mikos AG (2005) Flow perfusion culture of marrow stromal cells seeded on porous biphasic calcium phosphate ceramics. Ann Biomed Eng 33: 1238–1248
Isaksson H, Wilson W, van Donkelaar CC, Huiskes R, Ito K (2006) Comparison of biophysical stimuli for mechano-regulation of tissue differentiation during fracture healing. J Biomech 39: 1507–1516
Kelly DJ, Prendergast PJ (2006) Prediction of the optimal mechanical properties for a scaffold used in osteochondral defect repair. Tissue Eng 12: 1509–2519
Lacroix D, Château A, Ginebra MP, Planell JA (2006) Micro-finite element models of bone tissue-engineering scaffolds. Biomaterials 27: 5326–5334
Lacroix D, Planell JA, Prendergast PJ (2009) Computer-aided design and finite-element modelling of biomaterial scaffolds for bone tissue engineering. Phil Trans R Soc A 367: 1993–2009
Lacroix D, Prendergast PJ, Li G, Marsh D (2002) Biomechanical model to simulate tissue differentiation and bone regeneration: application to fracture healing. Med Biol Eng Comput 40: 14–21
Lesman A, Blinder Y, Levenberg S (2010) Modeling of flow-induced shear stress applied on 3D cellular scaffolds: implications for vascular tissue engineering. Biotechnol Bioeng 105: 645–654
Maes F, Van Ransbeeck P, Van Oosterwyck H, Verdonck P (2009) Modeling fluid flow through irregular scaffolds for perfusion bioreactors. Biotechnol Bioeng 103: 621–630
Martin I, Wendt D, Heberer M (2004) The role of bioreactors in tissue engineering. Trends Biotechnol 22: 80–86
Milan JL, Planell JA, Lacroix D (2009) Computational modelling of the mechanical environment of osteogenesis within a polylactic acid–calcium phosphate glass scaffold. Biomaterials 30: 4219–4226
Navarro M, Michiardi A (2008) The challenge of combining cells, synthetic materials and growth factors to engineer bone tissue. In: Barnes SJ, Harris LP (eds) Tissue engineering: roles, materials and applications. Novapublishers, New York, pp 19–66
Olivares A, Marsal E, Planell JA, Lacroix D (2009) Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials 30: 6142–6149
Porter B, Zauel R, Stockman H, Guldberg R, Fyhrie D (2005) 3-D computational modeling of media flow through scaffolds in a perfusion bioreactor. J Biomech 38: 543–549
Potier E, Noailly J, Ito K (2010) Directing bone marrow-derived stromal cell function with mechanics. J Biomech 43: 807–817
Prendergast PJ, Huiskes R, Søballe K (1997) Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech 30: 539–548
Raimondi MT, Moretti M, Cioffi M, Giordano C, Boschetti F, Lagana K, Pietrabissa R (2006) The effect of hydrodynamic shear stress on 3D engineered chondrocyte systems subject to direct perfusion. Biorheol 43: 215–222
Sandino C, Checa S, Prendergast PJ, Lacroix D (2010) Simulation of angiogenesis and cell differentiation in a CaP scaffold subjected to compressive load strains using a lattice modeling approach. Biomaterials 31: 2446–2452
Sandino C, Planell JA, Lacroix D (2008) A finite element study of mechanical stimuli in scaffolds for bone tissue engineering. J Biomech 41: 1005–1014
Sikavitsas VI, Temenoff JS, Mikos AG (2001) Biomaterials and Bone mechanotransduction. Biomaterials 22: 2581–2593
Stops AJF, Heraty KB, Browne M, O’Brien FJ, McHugh PE (2010) A prediction of cell differentiation and proliferation within a collagen-glycosaminoglycan scaffold subjected to mechanical strain and perfusive fluid flow. J Biomech 43: 618–626
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandino, C., Lacroix, D. A dynamical study of the mechanical stimuli and tissue differentiation within a CaP scaffold based on micro-CT finite element models. Biomech Model Mechanobiol 10, 565–576 (2011). https://doi.org/10.1007/s10237-010-0256-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-010-0256-0