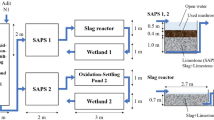
Abstract
Rehabilitation at a metal mine in New Zealand is complete with the exception of a 22 L/s discharge pumped from underground. The discharge has a pH of ≈6, alkalinity of ≈150 mg/L, dissolved oxygen (DO) <1 mg/L, elevated Fe and Mn, as well as elevated concentrations of Zn and As. Planning passive treatment was difficult because historical total Fe ranged from 20 to 200 mg/L, and Mn (11–22 mg/L) is soluble in the circumneutral pH range, due to conditions in the workings. Speciation analysis and modelling of the mine drainage chemistry indicated four factors were important for passive treatment design: (1) dissolved Fe is ≈20 mg/L and all is dissolved Fe(II); (2) Fe(II) concentration is stable because it is limited by saturation with respect to siderite (FeCO3); (3) the remaining Fe is colloidal Fe(OH)3 with a variable concentration; and (4) Mn is limited by saturation with respect to rhodochrosite (MnCO3). Equilibrium of Fe and Mn with minerals means that dissolved concentrations have an upper limit and are relatively stable, allowing an optimized treatment system. A pilot-scale passive treatment system was installed that included an oxygenation cascade of drops through V-notch weirs, settling ponds, and a slag leaching bed. Oxidation of Fe(II) to Fe(III) was followed by precipitation and settling of Fe(OH)3. Bicarbonate alkalinity in the mine drainage prevented acidification, and release of dissolved CO2 caused the pH to increase slightly. Manganese was removed by precipitation (of carbonates, oxides or oxy-hydroxides) in the slag leaching bed at elevated pH and high DO. Zinc and As were removed by adsorption onto Fe(OH)3. The oxygenation system removed 82–96 % of the Fe and 10 % of the Mn. The slag leaching bed removed 99 % of the remaining Mn.
Zusammenfassung
Die Renaturierung eines Erzbergwerkes in Neuseeland ist bis auf die Behandlung des abzupumpenden Grubenwassers (22 L/s) abgeschlossen. Das Grubenwasser hat einen pH-Wert von ≈6, eine Alkalinität von ≈150 mg/L CaCO3, gelösten Sauerstoff < 1 mg/L, hohe Konzentrationen an Fe und Mn sowie erhöhte Konzentrationen an Zn und As. Die Planung der passiven Behandlung war schwierig. Historische Messergebnisse für die Fe-Konzentration reichten von 20 bis zu 200 mg/L und Mn (11–20 mg/L) ist bei neutralem pH und den Bedingungen in den ehemaligen Bergwerksstollen und –schächten löslich. Die Analyse der chemischen Speziation der Metalle und die Modellierung des Chemismus des Grubenwassers ergaben vier Faktoren, die für die Gestaltung der passiven Grubenwasserbehandlung entscheidend waren: (1) der Anteil an gelöstem Fe beträgt ≈20 mg/L und ist vollständig Fe(II); (2) die Fe(II)-Konzentration ist stabil, da sie durch die Sättigung von Siderit (FeCO3) begrenzt ist; (3) weiteres Fe ist kolloidales Fe(OH)3 in variablen Konzentrationen; und (4) die Mn-Konzentrationen sind durch die Sättigung von Rhodochrosit (MnCO3) begrenzt. Die Gleichgewichte von Fe und Mn mit Mineralen bedeuten, dass die Konzentrationen von gelöstem Fe und Mn begrenzt sind und relativ stabil. Das erlaubt ein optimiertes Behandlungssystem. Eine Pilotanlage der passiven Behandlung wurde errichtet, die eine Oxidationskaskade aus V-förmig eingekerbten Rinnen, Absetzbecken und ein Schlackebett umfasst. Nach der Oxidation von Fe(II) zu Fe(III) erfolgte die Fällung und Sedimentation von Fe(OH)3. Die Alkalinität des Grubenwassers, bedingt durch Hydrogenkarbonat, verhinderte eine Versauerung. Die Abgabe von CO2 bewirkte eine leichte Anhebung des pH-Wertes. Mn wurde durch Fällung als Karbonat, Oxid oder Oxi-Hydroxid im Schlackebett bei erhöhtem pH-Wert und hoher Sauerstoffkonzentration zurückgehalten, Zn und As durch Adsorption an Fe(OH)3. Das Oxidationssystem bewirkte eine Rückhaltung von 82–96 % Fe und 10 % Mn. Das Schlackebett hielt 99 % des verbleibenden Mn zurück.
Resumen
La rehabilitación de una mina metalífera en Nueva Zelanda ha sido completa con la excepción de una descarga de 22 L/s bombeada desde la zona subterránea. La descarga tiene un pH de ≈6, alcalinidad de ≈150 mg/L, oxígeno disuelto (DO) < 1 mg/L, exceso de Fe y Mn, y elevadas concentraciones de Zn y As. La decisión sobre el tratamiento pasivo fue dificultosa porque las concentraciones de Fe total (históricamente se mantuvieron entre 20 y 200 mg/L) y de Mn (entre 11 y 22 mg/L) son solubles en el rango de pH cercano a la neutralidad. El análisis de especiación y el modelado de la química del drenaje de mina indicaron cuatro factores para el diseño del tratamiento pasivo: (1) Fe disuelto es ≈20 mg/L y existe como Fe(II); (2) la concentración de Fe(II) es estable porque está limitado por la saturación de siderita (FeCO3); (3) el Fe remanente es Fe(OH)3 coloidal con una concentración variable; y (4) Mn está limitado por la saturación de rodocrosita (MnCO3). Los equilibrios de Fe y Mn con los minerales implican un límite superior para las concentraciones que son relativamente estables, permitiendo un sistema optimizado de tratamiento. Se instaló un sistema de tratamiento pasivo a escala piloto que incluyó una cascada de oxigenación por gotas a través de vertederos en forma de V, estanques de sedimentación y un lecho fijo con escoria. La oxidación de Fe(II) a Fe(III) fue seguida por precipitación y sedimentación de Fe(OH)3. La alcalinidad del bicarbonato en el drenaje de mina previno la acidificación y la liberación de CO2 disuelto causó que el pH se incremente ligeramente. Mn fue removido por precipitación (de carbonatos, óxidos o oxohidróxidos) en el lecho fijo con escoria a pH elevado y alta DO. Zn y As fueron removidos por adsorción sobre Fe(OH)3. El sistema de oxigenación removió entre 82 y 96 % del Fe y 10 % del Mn. La lixiviación de escoria removió 99 % del Mn remanente.
摘要
除了从地下抽排的22 L/s的矿山废水外,某金属矿(新西兰)的采后治理已经完成。该金属矿山废水pH ≈6,碱度 ≈150 mg/L,溶解氧(DO) < 1 mg/L,含过量Fe、Mn,Zn、As浓度增高。受采场条件影响,矿山废水呈近中性,Fe(浓度20 mg/L ~ 200 mg/L)、Mn(浓度11 mg/L ~ 22 mg/L)多可溶态,给被动处理带来困难。矿山废水化学形态分析及水化学模拟结果表明,废水被动处理主要受4个因素影响:(1)溶解性Fe浓度为20 mg/L且均为Fe(II);(2)受菱铁矿(FeCO3)饱和度控制,Fe(II)浓度较稳定;(3)其余Fe为浓度不稳定的胶体态Fe(OH)3;(4)Mn浓度受菱锰矿(MnCO3)饱和度控制。Fe、Mn浓度因矿物溶解平衡存在稳定上限,为废水被动处理系统优化提供了条件。小规模的被动处理系统包括V型堰跌水曝气池、沉淀池和渣浸床。Fe(II) 被氧化为 Fe(III)后生成 Fe(OH)3沉淀。矿山废水中重碳酸盐碱度阻碍了废水进一步酸化,并释放出可溶CO2,pH值略增大。锰在渣浸床pH值升高和溶解氧(DO)增多的条件下生成碳酸盐、氧化物及氧氢氧化物沉淀。锌和砷被Fe(OH)3吸附。曝气系统的铁、锰去除率分别为82 % ~ 96 %和10 %。渣浸床的余锰去除率为99 %。














Similar content being viewed by others
References
Alarcon LE (1997) Long term mine site rehabilitation studies at Stockton open-cast coal-mine. Unpubl MSc Thesis in Geology, Univ of Canterbury, Christchurch, New Zealand
American Public Health Association (APHA) (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington
Bamforth SM, Manning DAC, Singleton I, Younger PL, Johnson KL (2006) Manganese removal from mine waters—investigating the occurrence and importance of manganese carbonates. Appl Geochem 21:1274–1287
Baylar A, Bagatur T (2000) Aeration performance of weirs. Water SA 26:521–526
Berger BR, Bethke PM (1985) Geology and geochemistry of epithermal systems. In: Robertson JM (ed), Rev Econ Geol, vol 2. ISBN: 978-1-629495-60-6
Bigham JM, Schwertmann U, Traina SJ, Winland RL, Wolf M (1996) Schwermannite and the chemical modeling of iron in acid sulphate waters. Geochim Cosmochim Acta 60(12):2111–2121
Black A, Trumm D, Lindsay P (2005) Impacts of coal mining on water quality and metal mobilisation: case studies from West Coast and Otago. In: Moore TA, Black A, Centeno JA, Harding JS, Trumm DA (eds) Metal contaminants in New Zealand, sources, treatments, and effects on ecology and human health. Resolutionz Press, New Zealand, pp 247–260
Boothroyd IKG, Goldstone A, Fitzpatrick M, MacGillivray R (2005) Criteria for the protection of aquatic ecological values at Golden Cross Mine: a case study. In: Moore TA, Black A, Centeno JA, Harding JS, Trumm DA (eds) Metal contaminants in New Zealand, sources, treatments, and effects on ecology and human health. Resolutionz Press, New Zealand, pp 359–374
Craw D, Brown K, Webster-Brown J (2005) Metals derived from gold mining and geothermal sources. In: Moore TA, Black A, Centeno JA, Harding JS, Trumm DA (eds) Metal contaminants in New Zealand, sources, treatments, and effects on ecology and human health. Resolutionz Press, New Zealand, pp 231–246
deJoux A (2003) Geochemical investigation and computer modelling of acid mine drainage, Sullivan mine, Denniston Plateau, West Coast. Unpubl MSc thesis in Geology, University of Canterbury, Christchurch, New Zealand
Faulkner BB, Skousen JG (1994) Treatment of acid mine drainage by passive treatment systems. In: Proceedings of international land reclamation and mine drainage conference, US Bureau of Mines SP 06A-94, Pittsburgh, PA, USA
Geroni JN, Cravotta CA, Sapsford DJ (2012) Evolution of the chemistry of Fe bearing waters during CO2 degassing. Appl Geochem 27:2335–2347. doi:10.1016/j.apgeochem.2012.07.017
Haffert L, Craw D (2010) Geochemical processes influencing arsenic mobility at Bullendale historic gold mine, Otago, New Zealand. N Z J Geol Geophys 53:129–142
Hellier WW (1999) An integrated design model for passive treatment systems to abate water pollution from post-mining discharges (Ohiopyle State Park). In: Proceedings of National Association of Abandoned Mine Land Programs
Henley RW, Truesdell AH, Barton Jr. PB, Whitney JA (eds) (1984) Fluid-mineral equilibria in hydrothermal systems. In: Robertson JM (ed), Rev Econ Geol, vol 1. ISBN: 978-1-629495-59-0
James T (2003) Water quality of streams draining various coal measures in the North-central West Coast. In: Proceedings of the Australasian Institute of Mining and Metallurgy New Zealand Branch 36th annual conference, pp 103–114
Kim J, Walters R (2001) Oxygen transfer at low drop weirs. J Environ Eng 127:604–610
Koduri S, Barkdoll B (2003) Evaluation of oxygen transfer at stepped cascade aerators. In: Proceedings of World Water Congress
MacGillivray R, Maton T, Goldstone A (2001) Rehabilitation of the Golden Cross Mine, Coromandel, New Zealand. N Z Min 28:25–28
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: USGS WRI Report 99-4259, Denver, CO, USA
Plumlee G, Logsdon M (eds) (1999) The environmental geochemistry of mineral deposits, Part A: processes, techniques and health issues. Society of Economic Geologists, Chelsea
Pope J, Newman N, Craw D (2006) Coal mine drainage geochemistry, West Coast, South Island—a preliminary water quality hazard model. In: Proceedings of Australasian Institute of Mining and Metallurgy New Zealand Branch 39th annual conference, Waihi, New Zealand
Pope J, Newman N, Craw D, Trumm D, Rait R (2010) Factors that influence coal mine drainage chemistry, West Coast, South Island, New Zealand. J Geol Geophys 53:115–128
Rose AW, Cravotta CA III (1998) Geochemistry of coal mine drainage. In: Brady KBC, Smith MW, Schueck J (eds) Coal mine drainage prediction and pollution prevention in Pennsylvania. PA Department of Environmental Protection, Harrisburg, pp 1-1–1-22
Simmons J, Ziemkiewicz P, Black DC (2002) Use of steel slag leach beds for the treatment of acid mine drainage. Mine Water Environ 21:91–99
Skousen JG (2002) A brief overview of control and treatment technologies for acid mine drainage. In: Proceedings of National Mtg of the American Society of Mining and Reclamation, pp 879–899. http://www.asmr.us/Publications/Conference%20Proceedings/2002/0879%20Skousen.pdf
Skousen JG, Sextone A, Ziemkiewicz PF (2000) Acid mine drainage control and treatment. In: Barnhisel RI, Darmody RG, Daniels L (eds) Reclamation of drastically disturbed lands, agronomy monograph no 41. American Society of Agronomy, Madison, pp 131–168
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York
Trumm D (2010) Selection of active and passive treatment systems for AMD—flow charts for New Zealand conditions. NZJ Geol Geophys 53(2–3):195–210
Watzlaf GR, Hyman DM (1995) Limitations of passive systems for the treatment of mine drainage. In: Proceedings of 17th annual conference of the National Association of Abandoned Mine Lands, French Lick, IN, USA
Webster-Brown J, Craw D (2005) Examples of trace metal mobility around historic and modern mines. In: Moore TA, Black A, Centeno JA, Harding JS, Trumm DA (eds) Metal contaminants in New Zealand, sources, treatments, and effects on ecology and human health. Resolutionz Press, New Zealand, pp 213–230
Younger PL, Banwart SA, Hedin RS (2002) Mine water: hydrology, pollution, remediation. Kluwer Academic Publication, Dordrecht
Ziemkiewicz P, Skousen J (1998) The use of steel slag in acid mine drainage treatment and control. In: Proceedings of 19th annual West Virginia Surface mining drainage task force symposium, Morgantown, WV, USA. http://wvmdtaskforce.com/proceedings/98/98ZIE/98ZIE.HTM
Acknowledgments
We acknowledge the mining company that funded the construction and operation of the treatment system, and allowed the results to be published. Detailed analysis, write up, and publication of this data was financed by the Ministry for Business, Innovation and Employment.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure l
Fe Eh–pH diagram for speciation of Fe in mine pool water based on pH and ORP measurements (n = 124) (PDF 13 kb)
Supplemental Fig. 2
Mn Eh–pH diagram for speciation of Mn in mine pool water based on pH and ORP measurements (n = 124) (PDF 12 kb)
Rights and permissions
About this article
Cite this article
Trumm, D., Pope, J. Passive Treatment of Neutral Mine Drainage at a Metal Mine in New Zealand Using an Oxidizing System and Slag Leaching Bed. Mine Water Environ 34, 430–441 (2015). https://doi.org/10.1007/s10230-015-0355-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-015-0355-3