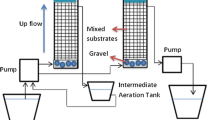
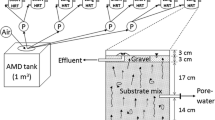
Abstract
Microcosm studies in the laboratory demonstrate that sufficient dosages of wastewater effluent (microbial inoculum) and returned milk (substrate) can effectively raise the pH of pyrite-amended acid mine drainage water to circumneutral levels under aerobic conditions in as little as 7 days, and the pH remains at these levels for >19 months. Microbial analysis indicates that a complex biofilm (>70 species) forms over the pyrite. The biofilm dominantly consists of facultative anaerobes, which potentially interact with obligate anaerobes, such as sulfate-reducing Desulfosporosinus sp., to maintain an oxygen-free micro-environment surrounding the pyrite, even though the overlying water remains aerobic. The biofilm became established in water samples with an initial pH as low as 2, and subsequently caused the water pH to increase to circumneutral levels. Concurrently, concentrations of Al, As, Cu, Fe, Pb, Ni, and Zn all decreased substantially compared to baseline concentrations in the control microcosms.







Similar content being viewed by others
References
Abou-Shanab RA, Delorme TT, Angle SJ, Chaney RL, van Berkum P, Ghozlan HA, Ghanem K, Moawad HA (2003) Molecular characterization and identification of nickel-resistance soil bacteria in the rhizosphere of Alyssum murale. Unpubl GenBank entry AY512827
Adams DJ, Gardner KR, Davidson RA, Esplin DN, Pickett TM, Heyrend TT, Montgomery JR (1995) Biotechnology for pollution prevention in the mining industry. Presented at the North West Mining Association Open Industry Briefing, Spokane, WA, USA
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Batten KM, Scow KM (2003) Sediment microbial community composition and methylmercury pollution at four mercury mine-impacted sites. Microb Ecol 46:429–441
Canty M (1998) Overview of the sulfate-reducing bacteria demonstration project under the Mine Waste Technology Program. Miner Process Extr Metall Rev 19:61–80
Carlson L, Bigham JM, Schwertmann U, Kyek A, Wagner F (2002) Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: a comparison with synthetic analogues. Environ Sci Technol 36:1712–1719
Chang IS, Shin PK, Kim BH (2000) Biological treatment of acid mine drainage under sulphate-reducing conditions with solid waste materials as substrate. Water Res 34:1269–1277
Characklis WG (1990) Laboratory biofilm reactors. In: Characklis WG, Marshall KC (eds) Biofilms. Wiley, New York, pp 55–89
Christensen BE, Characklis WG (1990) Physical and chemical properties of biofilms. In: Characklis WG, Marshall KC (eds) Biofilms. Wiley, New York, pp 93–130
Coulton R, Bullen C, Hallet C (2003) The design and optimization of active mine water treatment plants. Land Contam Reclamat 11:273–279
Doye I, Duchesne J (2005) Column leaching test to evaluate the use of alkaline industrial wastes to neutralize acid mine tailings. J Environ Eng 131:1221–1229
Dvorak DH, Hedin RS, Edenborn HM, McIntire PE (1992) Treatment of metal-contaminated water using bacterial sulfate reduction: results from pilot scale reactors. Biotechnol Bioeng 40:609–616
Elliot P, Ragusa S, Catcheside D (1998) Growth of sulfate-reducing bacteria under acidic conditions in an anaerobic bioreactor as a treatment system for acid mine drainage. Water Res 32:3724–3730
Elsetinow AR, Borda MJ, Schoonen MAA, Strongin DR (2003) Suppression of pyrite oxidation in acidic aqueous environments using lipids having two hydrophobic tails. Adv Environ Res 7:969–974
Fallgren P, Jin S (2005) Source control treatment of acid mine drainage utilizing sulfate-reducing bacteria. Presented at the Joint International Symp for Subsurface Microbiology (ISSM 2005) and Environmental Biogeochemistry (ISEB XVII), Jackson, WY, USA
Gill JJ, Sabour PM, Gong JH, Yu H, Leslie KE, Griffiths MW (2006) Characterization of bacterial populations recovered from the teat canals of lactating dairy and beef cattle by 16S rRNA gene sequence analysis. FEMS Microbiol Ecol 56:471–481
Ingvorsen K, Nielsen MY, Joulian C (2003) Kinetics of bacterial sulfate reduction in an activated sludge plant. FEMS Microbiol Ecol 46:129–137
Johnson DB, Dziurla M-A, Kolmert A, Hallberg KB (2002) The microbiology of acid mine drainage: genesis and biotreatment. S Afr J Sci 98:249–255
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Jong T, Parry DL (2003) Removal of sulfate and heavy metals by sulfate reducing bacteria in short-term bench scale upflow anaerobic packed bed reactor runs. Water Res 37:3379–3389
Keeney DR, Nelson DW (1982) Nitrogen–inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2, chemical and microbiological properties-agronomy monograph, vol 9, 2nd edn. ASA-SSSA, Madison, pp 643–698
Kim SD, Kilbme JJ, Cha DK (1999) Prevention of acid mine drainage by sulfate reducing bacteria: organic substrate addition to mine waste piles. Environ Eng Sci 16:139–145
Kjeldsen KU, Joulian C, Ingvorsen K (2004) Oxygen tolerance of sulfate-reducing bacteria in activated sludge. Environ Sci Technol 38:2038–2043
Kleinmann RLP, Hedin RS, Nairn RW (1998) Treatment of mine drainage by anoxic limestone drains and constructed wetlands. In: Geller A, Klapper H, Salomons W (eds) Acidic mining lakes: acid mine drainage, limnology and reclamation. Springer, Berlin, pp 303–319
Kusel KA, Roth U, Trinkwalter T, Peiffer S (2001) Effect of pH on the anaerobic microbial cycling on sulfur in mining-impacted freshwater lake sediments. Environ Exp Bot 46:213–223
Lens PN, de Poorter M-P, Cronenberg CC, Verstraete WH (1995) Sulfate reducing and methane producing bacteria in aerobic wastewater treatment systems. Water Res 29:871–880
Levings CD, Barry KL, Grout JA, Piercey GE, Marsden AD, Coombs AP, Mossop B (2004) Effects of acid mine drainage on the estuarine food web, Britannia Beach, Howe Sound, British Columbia, Canada. Hydrobiologia 525:185–202
Lyew D, Knowles R, Sheppard J (1994) The biological treatment of acid mine drainage under continuous flow conditions in a reactor. Trans I Chem E 72(B):42–47
Machemer SD, Wildeman TR (1992) Adsorption compared with sulfide precipitation as metal removal processes from acid mine drainage in a constructed wetland. J Contam Hydrol 9:115–131
Manz W, Eisenbrecher M, Neu TR, Szewzyk U (1998) Abundance and spatial organization of Gram-negative sulfate-reducing bacteria in activated sludge investigated by in situ probing with specific 16S rRNA targeted oligonucleotides. FEMS Microbiol Ecol 25:43–61
Muyzer G, Dewaal EC, Uitterlinden AG (1993) Profiling of complex microbial-populations by denaturing gradient gel-electrophoresis analysis of polymerase chain reaction-amplified genes-coding for 16s ribosomal-RNA. Appl Environ Microbiol 59:695–700
Purohit HJ, Kapley A (2005) Microbial diversity in a UASB reactor treating wastewater from a CETP operating in high salinity. Unpubl GenBank entry DQ345944
Schramm A, Santegoeds CM, Nielsen HK, Ploug H, Wagner M, Pribyl M, Wanner J, Amann R, de Beer D (1999) On the occurrence of anoxic microniches, denitrification, and sulfate reduction in aerated activated sludge. Appl Environ Microbiol 65:4189–4196
Skousen J, Rose A, Geidel G, Foreman J, Evans R, Hellier W (1998) Handbook of technologies for avoidance and remediation of acid mine drainage. The National Mine Land Reclamation Center, Morgantown, 131 pp
Tabak HH, Scharp R, Burckle J, Kawahara FK, Govind R (2003) Advances in biotreatment of acid mine drainage and biorecovery of metals: 1. Metal precipitation for recovery and recycle. Biodegradation 14:423–436
Tuttle JH, Dugan PR, Randles CI (1969) Microbial sulfate reduction and its potential as an acid mine water pollution abatement procedure. Appl Environ Microbiol 17:297–302
Van Houten RT, Hulshoff Pol LW, Lettinga G (1994) Biological sulphate reduction using gas lift reactors fed with hydrogen and carbon dioxide as energy and carbon sources. Biotechnol Bioeng 44:586–594
Webb JS, McGinness S, Lappin-Scott HM (1998) Metal removal by sulphate-reducing bacteria from natural and constructed wetlands. J Appl Microbiol 84:240–248
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S Ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Zhang X, Borda MJ, Schoonen MAA, Strongin DR (2003a) Pyrite oxidation inhibition by a cross-linked lipid coating. Geochem Trans 4:8–11
Zhang X, Borda MJ, Schoonen MAA, Strongin DR (2003b) Adsorption of phospholipids on pyrite and their effect on surface oxidation. Langmuir 19:8787–8792
Zhang X, Kendall TA, Hao J, Strongin DR, Schoonen MAA, Martin ST (2006) Physical structures of lipid layers on pyrite. Environ Sci Technol 40:1511–1515
Acknowledgments
The authors thank Dr. Terry Brown (Western Research Institute) for his technical advice, Dr. Amy Pruden (Colorado State University) for assistance in microbial analysis, and Jeff Cooper, Joel Mason, and Jesse Newcomer (Western Research Institute) for assistance with this project. This research was conducted and supported by Western Research Institute (WRI) and Kennecott Energy. Financial support was provided by the U.S. Dept of Energy (DoE) through Western Research Institute’s Cooperative Agreement DE-FC26-98FT40322 with DoE and Kennecott Energy. Any opinions, findings, conclusions, or recommendations expressed herein are those of the authors and do not reflect the view of the DoE or Kennecott Energy.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10230-008-0043-7
Rights and permissions
About this article
Cite this article
Jin, S., Fallgren, P.H., Morris, J.M. et al. Biological Source Treatment of Acid Mine Drainage Using Microbial and Substrate Amendments: Microcosm Studies. Mine Water Environ 27, 20–30 (2008). https://doi.org/10.1007/s10230-007-0026-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-007-0026-0