Abstract
The current and cascading effects of global change challenges the interactions both between animal individuals (i.e. social and sexual behaviour) and the environment they inhabit. Amphibians are an ecologically diverse class with a wide range of social and sexual behaviours, making them a compelling model to understand the potential adaptations of animals faced with the effects of human-induced rapid environmental changes (HIREC). Poison frogs (Dendrobatoidea) are a particularly interesting system, as they display diverse social behaviours that are shaped by conspecific and environmental interactions, thus offering a tractable system to investigate how closely related species may respond to the impacts of HIREC. Here, we discuss the potential impacts of global change on poison frog behaviour, and the future challenges this group may face in response to such change. We pay special attention to parental care and territoriality, which are emblematic of this clade, and consider how different species may flexibly respond and adapt to increasingly frequent and diverse anthropogenic stress. More specifically, we hypothesise that some parents may increase care (i.e. clutch attendance and distance travelled for tadpole transport) in HIREC scenarios and that species with more generalist oviposition and tadpole deposition behaviours may fare more positively than their less flexible counterparts; we predict that the latter may either face increased competition for resources limited by HIREC or will be forced to adapt and expand their natural preferences. Likewise, we hypothesise that human-driven habitat alteration will disrupt the acoustic and visual communication systems due to increased noise pollution and/or changes in the surrounding light environment. We highlight the need for more empirical research combining behavioural ecology and conservation to better predict species’ vulnerability to global change and efficiently focus conservation efforts.
Similar content being viewed by others
Introduction
Environmental changes, including shifting continents and climatic fluctuations, have been shown to prompt diverse responses in organisms across a wide range of taxa (Ricklefs and Schluter 1993; Rosenzweig 1995) throughout evolutionary time. However, the unprecedented scale and pace of recent and current human-induced rapid environmental changes (HIREC), such as habitat destruction/fragmentation (Pimm and Raven 2000), climate change (Parmesan and Yohe 2003), and exposure to novel biotic (e.g. exotic species, pathogens, and parasites: Lockwood et al. 2013) and abiotic (e.g. environmental pollutants: Rohr et al. 2006) stressors, represent new challenges for many species which have not experienced such rapid changes in their evolutionary past (Palumbi 2001). The impact of HIREC on the natural world is colossal (Wake and Vredenburg 2008; Cowie et al. 2022), affecting the availability of important resources (i.e. food and shelter; Fahrig 2003), altering conspecific and heterospecific interactions (Tuomainen and Candolin 2011; Candolin and Wong 2012), and ultimately threatening many species and populations (Pimm and Raven 2000; Wake and Vredenburg 2008; Cowie et al. 2022).
For many animals, survival and reproduction in rapidly changing environments are expected to be shaped by the plasticity of their behavioural responses (Hendry et al. 2008; Sih et al. 2011; Sih 2013; Wong and Candolin 2015). Sometimes, behavioural changes may be enough for an individual to adapt to new conditions or can provide additional time for genetic adaptation to occur (Pigliucci 2001). For example, great tits (Parus major) in urban environments have learnt to adjust their song frequency to avoid interference from city noise (Slabbekoorn and Peet 2003), while northern quolls (Dasyurus hallucatus) in Australia have learnt to avoid eating highly-toxic invasive cane toads (Rhinella marina) (Kelly and Phillips 2017). However, species can also show maladaptive responses in HIREC scenarios, such as sea turtle hatchlings following artificial light instead of natural cues (Tuxbury and Salmon 2005), or aquatic insects ovipositing on asphalt or glass that resembles the surface of the water (Kriska et al. 1998, 2008), causing serious population declines (Tuomainen and Candolin 2011; Robertson et al. 2013). In other cases, behavioural changes can determine which individuals will survive and reproduce under novel conditions, acting as a driving force in evolutionary processes (West-Eberhard 2003; Crispo 2007; Tuomainen and Candolin 2011). Thus, changes in behaviour will directly influence how species evolve under HIREC.
While individual behavioural responses will affect population dynamics on a local scale, the effect of HIREC on sociality and interspecific interactions has far-reaching ecological implications for broader community dynamics. Environmental changes can directly and indirectly influence the way in which individuals interact, not only with other species (e.g. predators and prey, hosts and parasites) but also with each other (Candolin and Wong 2012). Social interactions, ranging from choosing a mate to providing offspring with care, can be affected by HIREC in multiple ways (Croft et al. 2008). For example, ship noise reduces the ability of Lusitanian toadfish (Halobatrachus didactylus) to detect conspecific acoustic signals, essential for mate attraction (Vasconcelos et al. 2007), while water turbidity reduces male-male competition in three-spined stickleback (Gasterosteus aculeatus), compromising the honesty of agonistic signals, which are relevant indicators of parenting ability (Wong et al. 2007). Similarly, human disturbance can directly reduce the nest attendance of bearded vultures (Gypaetus barbatus), increasing the probability of breeding failure (Arroyo and Razin 2006).
Social interactions have a critical effect on individual fitness (Allee et al. 1949) and, consequently, on population dynamics. Parental care, for example, is known to enhance the offspring’s fitness by increasing their survival, growth and/or quality, and, ultimately, their lifetime reproductive success (Royle et al. 2012). Despite the obvious benefits for the offspring, parental care comes at a cost to the caregiver in the form of energy expenditure, loss of mating opportunities, and increased predation risk while tending their young (Alonso-Alvarez et al. 2012). Thus, an individual’s investment in parental care depends on the value of their existing offspring in relation to future reproductive opportunities (Alonso-Alvarez et al. 2012; Royle et al. 2012). Under changing environmental conditions, both the energetic costs of care for the parents and the fitness benefits for the young could be altered, influencing population recruitment (Alonso-Alvarez et al. 2012; Ratikainen et al. 2018). In the face of low resource availability, parents can, for instance, reduce their current investment into offspring with the expectation of better reproductive opportunities in the future (Winkler 1987). This decline in care quality, in turn, can cause long-term changes in the behaviour of the offspring, including aggressiveness and boldness (Armstrong 2019), cognition (Bredy et al. 2004), and F1’s parental behaviour (Gromov 2009). Research conducted in songbirds, for example, has shown that nutritional stress during early development stages (when songbirds depend on their parents for food), negatively affects brain development and male song quality in adulthood (Nowicki et al. 2002). In rodents, offspring who are groomed less frequently during early postnatal periods exhibit lower spatial learning and memory in adulthood (Liu et al. 2000; Bredy et al. 2004). Decreased investment in the face of challenging environmental conditions is not the rule, however, as some parents appear to increase their workload in the face of sub-optimal conditions (Vincze et al. 2017). Ultimately, the adaptability of parental behaviour/cooperation appears to be the most accurate predictor of species successfully overcoming the varied pressures of global change in the wild (Vincze et al. 2017).
Behavioural responses to global change largely differ between species. Amphibians are excellent models to study such responses owing to their broad range of social behaviours and their wide distribution across latitudes and climates, being found in all continents except Antarctica. Furthermore, their key position in trophic webs, their role as sentinel species and bioindicators of ecosystem health thanks to their sensitivity to environmental changes, and their dramatic decline around the globe (Hopkins 2007) make them a useful system to study the impact of human disturbances. In fact, amphibians are considered the most threatened vertebrate class on the planet (Stuart et al. 2004; Wake and Vredenburg 2008; Nori et al. 2015; IUCN 2020; Cordier et al. 2021), primarily due to habitat fragmentation/destruction and the spread of a pathogenic fungus (Daszak et al. 2003; Pounds et al. 2006; Cordier et al. 2021).
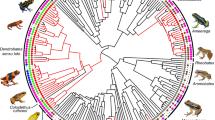
One of the most emblematic and well-studied groups of amphibians showing complex and diverse social behaviours are Neotropical poison frogs (Dendrobatidae) and their closest relatives (Aromobatidae), altogether referred to as the superfamily Dendrobatoidea (Cope 1865; Grant et al. 2006) and hereon referred to as ‘poison frogs’ for simplicity. Distributed from Nicaragua in Central America to Bolivia in South America, poison frogs generally inhabit tropical rainforests (Summers and Tumulty 2014), often in areas that are under severe degradation, and exhibit a large diversity in mating systems, parental care strategies, and communication modalities (reviewed in Summers and Tumulty 2014). Males generally defend long-term territories from conspecifics through so-called advertisement calls and, if necessary, physical combat (Fig. 1A; Pröhl 2005). Defending these territories is often crucial to male reproductive success, as courtship, mating, and oviposition take place therein (Pröhl 2005). In most species, males perform parental care, which consists of clutch attendance and larval transport (Fig. 1B) from terrestrial oviposition sites (e.g. leaf litter and leaves on bushes) to water bodies such as streams, temporary ponds, or small pools of water formed in plant structures (i.e. phytotelmata) (Summers and Tumulty 2014). Tadpoles are confined in these water bodies until completing metamorphosis (Weygoldt 1987; Lehtinen et al. 2004; Summers and McKeon 2004; Schulte et al. 2020). While uniparental male care is the basal reproductive strategy in poison frogs (Weygoldt 1987; Carvajal-Castro et al. 2021), multiple lineages have evolved biparental or exclusive female care, where females transport tadpoles (Fig. 1C) and feed them with unfertilised trophic eggs (Summers et al. 1999a). The transition to female or biparental care has been suggested to be the result of using small phytotelmata with scarce food resources (Brown et al. 2010; Carvajal-Castro et al. 2021), and biparental care has been proposed as the precursor of monogamy (Brown et al. 2008, 2010; Summers and Tumulty 2014; Tumulty et al. 2014). It is precisely the interaction between the diverse sexual and social systems of poison frogs, combined with the pressing effects of HIREC, that makes this group a relevant model through which to test and understand the impacts of global change.
Poison frogs and their unique social behaviours may be impacted by global change. A Males of Dendrobates tinctorius engaged in physical combat, where often one male pushes, kicks, and gets on top of the other trying to press them against the substrate; B male Ameerega hahneli transporting his tadpoles (pointed at by the arrow) to a body of water; C tadpole (pointed at by the arrow) transport is done by females in Oophaga granulifera; D habitat disturbance can alter the way in which colours are perceived by con- and heterospecifics, as shown in O. pumilio, and thus affect communication systems; E males of D. tinctorius are in charge of clutch (pointed at by the arrow) attendance; F climate change can increase the risk of tadpole death (agonising tadpoles pointed at by the arrows) by desiccation of nurseries; G Ranitomeya ventrimaculata parents (pointed at by the dashed arrows) lay clutches (pointed at by the solid arrow) in bromeliads occupied by a large tadpole in periods of low rainfall to increase the survival probabilities of the tadpole therein; H O. lehmanni is highly threatened due to illegal pet trade activities. Males are thought to be more likely to be found by collectors because of their vocalisations (see the inflated vocal sac pointed by the arrow); I Andinobates bombetes adjusts their calling behaviour to avoid interference caused by traffic noise. Photo credits: Bibiana Rojas (A, B, C, E, F, G); Justin P. Lawrence (D); Mileidy Betancourth (H); Fernando Vargas (I)
Although global change is expected to influence social behaviours in several ways, surprisingly little is known about how these effects take place in wild populations of poison frogs. Moreover, most studies analyse environmental stressors independently, often underseeing potential interactions and synergic effects. For example, while tadpoles manage to cope with predator-induced stress and low concentrations of pesticides separately, when exposed to both at the same time they show substantial mortality (Relyea and Mills 2001). Further research combining animal behaviour and conservation biology (Caro 1999) is necessary to identify species-specific relevant HIREC and to understand how they may adapt (or not) their behaviours accordingly. Only by doing so, we may be able to evaluate populations’ vulnerability to global change, develop predictive models and focus conservation efforts (Schroeder et al. 2011). Here, we illustrate key points about the potential impacts of, and responses to, HIREC using Neotropical poison frogs’ social behaviours as a model system. We specifically focus on territoriality and parental care behaviours, as they could be of special importance due to their capacity to buffering offspring against HIREC. Using this information as a baseline, we identify knowledge gaps and formulate new testable hypotheses to assess (1) the nature and magnitude of HIREC impact on wild populations of poison frogs, and (2) potential parental care and aggression responses to these HIREC.
Impacts of global change on poison frogs
Poison frogs depend on a wide variety of microhabitats in different life stages. Leaf litter and phytotelmata, for example, serve as primary breeding sites, shelters, and nurseries for poison frogs; in addition to being defendable resources for territorial species, they provide more stable temperature and humidity conditions than open areas with little canopy (Duellman and Trueb 1994). The dependence on suitable microhabitats together with the obligate use of small water bodies for reproduction or development make many Neotropical frogs particularly vulnerable to HIREC (Donnelly and Crump 1998; Touchon and Warkentin 2009).
Habitat loss and climate change
Many tropical regions are subject to unprecedented rates of habitat loss (Lewis et al. 2015; Taubert et al. 2018). Over the last decade, deforestation patterns in the Amazonian rainforest have switched from localised large forest clearings to geographically spread small-scale deforestation events driven by agricultural intensification, land-use change, and natural resource exploitation (i.e. mining and logging activities) (Grau and Aide 2008; Hugo 2008; Kalamandeen et al. 2018). Small-scale deforestation pressures are expected to affect more remote areas and populations. This type of deforestation is also recognised as one of the main causes of more frequent and intense anomalies in the Amazonian hydrological cycle, such as extreme weather events (i.e. El Niño Southern Oscillation, hereafter El Niño) and dry spells during the rainy season (Lovejoy and Nobre 2018), which may be further exacerbated by global warming (Jiménez-Muñoz et al. 2016). Both the loss of habitat and more frequent climatic anomalies can affect poison frogs in multiple ways throughout their life stages, potentially leading to different behavioural responses and adaptations.
Disruption in communication systems
Habitat alteration through small-scale deforestation can directly affect conspecific communication in two different ways. First, because human-made gaps are known to have increased radiation and higher temperatures than other areas of the forest (Vitt et al. 1998), male calling behaviour can become unsustainable over long periods of time. This is because, in degraded conditions, males would be more exposed and thus could incur higher evaporative water loss and potential overheating. These physiological stressors entail behavioural consequences as, in the mid-to-long term, males would be unable to devote as much time to attract females and advertise territory ownership. Second, variations in the forest’s light environment can make an animal’s appearance change too (Endler 1993), which has been proven crucial in the courtship behaviour of some lekking bird species (Théry and Endler 2001). The detectability of the variable colour patterns found in D. tinctorius, likewise, has been shown to differ depending on whether they are seen under an open or closed canopy (Rojas et al. 2014). While this has been studied mostly in the context of predator–prey interactions, such differences in detectability in response to the surrounding light environment could be particularly relevant for species in which colour patterns play a role in mate choice (e.g. O. pumilio: Summers et al. 1999b; Maan and Cummings 2008; Yang et al. 2019) or underlie differences in other behavioural patterns such as boldness or aggressiveness (e.g. O. pumilio: Rudh et al. 2013; Pröhl and Ostrowski 2011; Crothers and Cummings 2015; O. granulifera: Willink et al. 2013, 2014). Importantly, human-driven habitat disturbance may not only affect the light environment but also the structure of the forest floor, which can alter detectability and visual contrast, thus causing potential interference in communication between conspecifics (Barnett et al. 2021). Furthermore, because poison frog colouration is partly based on carotenoid pigments acquired through the diet (Twomey et al. 2020), changes in the prey community availability driven by habitat disturbances could also alter the colouration of individuals. In fact, several studies have shown that a diet rich in carotenoids can indeed produce changes in colouration (Brenes-Soto and Dierenfeld 2014; Umbers et al. 2016; Stückler et al. 2022) and increase the reproductive success of captive frogs (Ogilvy et al. 2012; Dugas et al. 2013). Thus, changes in prey availability could affect the intake of carotenoids or their precursors and, in turn, affect intraspecific communication, particularly in species where colouration plays an important role in mate selection, as mentioned above.
Increased care and aggression under HIREC: a parent’s perspective
Reduced vegetation cover and longer dry spells could result in higher egg mortality due to dehydration, especially for amphibian species with nonaquatic eggs (Touchon and Warkentin 2009). For example Delia et al. (2013) found that offspring of the glass frog Hyalinobatrachium fleischmanni, a species with parental care, had higher mortality rates in years of low rainfall. Similar situations could arise in poison frogs due to the high susceptibility of their terrestrial clutches to evaporative water loss; in Allobates paleovarzensis, for instance, only 8.6% of the clutches survived until the transporting stage following an El Niño event compared to ~ 70% survival during a standard season (Rocha et al. 2021).
There are several behaviours that may help adult poison frogs reduce the vulnerability of their eggs to HIREC. On the one hand, choosing suitable oviposition sites is particularly important if larvae are unable to leave these sites when conditions become unfavourable. For example, in the tree frog Dendropsophus ebraccatus, a unique species which can flexibly choose between aquatic and nonaquatic deposition sites, changes in rainfall patterns since 1972 have altered oviposition-site selection (Touchon 2012). Although egg mortality was generally higher in aquatic sites due to greater predation risk, altered rainfall patterns driven by climate change increased clutch dehydration risk, shifting the optimal site choice by parents from terrestrial to aquatic habitats over the span of only 40 years. D. ebraccatus clearly provides an excellent system to measure the success of the adaptive decision-making by parents; however, whether or not poison frogs are as flexible in their use of oviposition and tadpole deposition sites requires further research. For terrestrially-breeding frogs, buffering the negative effects of HIREC could largely depend on the parents’ capacity to select specific microhabitats with favourable structures. Dendrobates tinctorius, for example, is a terrestrial-breeding frog with clutch attendance (Fig. 1D) and uniquely flexible deposition choices compared to other species that also use ephemeral pools as nurseries. D. tinctorius fathers transport tadpoles to diverse pools that range enormously in their vertical position (0– > 20 m), size (19 mL to − 270 L), and chemical composition (pH = 3 to − 7) (Fouilloux et al. 2021). We hypothesise that, when faced with the pressures of HIREC, species that can access (and tolerate) a wider variety of nurseries will fare better than those with narrower options. Species with flexible behaviour may also benefit from modulating care investment based on climatic conditions, e.g. when desiccation risk is high parents spend additional effort accessing especially deep/stable nurseries compared to potentially more relaxed, “riskier” choices throughout a consistently rainy season. Furthermore, we predict sites with denser canopy cover as well as abundant leaf litter and vegetal structures (e.g. fallen branches and hollow trunks) to provide more stable microclimate conditions for successful egg development. Nevertheless, different microhabitats may be weighed differently depending on species-specific biological and life-history requirements. Therefore, a better understanding of the microhabitat use of species both in undisturbed and disturbed areas is essential to implement effective conservation efforts.
On the other hand, to compensate for adverse environmental conditions, parents may adjust the intensity and frequency of clutch attendance to guarantee offspring survival (see examples in invertebrates (Dick et al. 1998), fish (Green and McCormick 2005), reptiles (Stahlschmidt and DeNardo 2010), and birds (Vincze et al. 2017)). Males of H. fleischmanni, for example, increase both the frequency and time spent on egg care in response to a reduction in relative humidity (Delia et al. 2013). One of the most common ways anurans provides egg attendance is by placing their body over the eggs to reduce evaporative water loss or directly moistening the eggs through physical contact with the ventral integument (Wells 2010). Although this behaviour has been suggested for some poison frogs (Souza et al. 2017), it is not ubiquitous across the family (Rocha et al. 2021). Furthermore, some amphibians can increase the amount of glycoprotein-rich jelly cores, jelly layers, or matrices surrounding the clutches, which protect embryos from dehydration and predators (Delia et al. 2020). So far, little research has focused on the potential egg attendance plasticity that poison frogs may present under environmental stress. Considering that egg attendance conflicts with other fitness-related activities, such as foraging and mating (e.g. Delia et al. 2013), investigating the trade-offs of parental decisions under environmental changes is essential to predict population dynamics. Therefore, if the costs of maintaining the current clutch surpass their fitness benefits, we would predict individuals to reduce their parental care effort or even abandon clutches completely, as seen in other taxa (e.g. birds: Bustnes and Erikstad 1991; Öberg et al. 2015; fish: Suski and Ridgway 2007).
It is noteworthy that in territorial species, such as most dendrobatid frogs (Pröhl 2005), the trade-off between defending territories and attending multiple clutches simultaneously may become magnified under habitat loss. Habitat loss and fragmentation can modify species movement as well as the availability of resources and suitable territories (Fahrig 2003), which can alter the carrying capacity of the area in different ways. Firstly, habitat fragmentation could reduce population density if edge effects are negative (e.g. increased predation pressure), or if the fragmented habitat is not able to sustain larger populations (Mullu 2016). Given that the costs of mate search in females depend on the number of suitable mates available, a lower population density could detract energy and time from tadpole care in species with female egg-feeding and tadpole transport. By contrast, the remaining patches of habitat could also increase population density by concentrating the surviving individuals from the disturbed habitat (Mullu 2016). In the resulting smaller and densely packed habitat patches, aggression rates between highly territorial individuals may increase due to a higher number of encounters and more competition for limited resources and territories (Fisher et al. 2021). In male tree lizards (Urosaurus ornatus), for instance, aggressive interactions between individuals are more frequent in resource-limited burned sites than in resource-rich habitats (Lattanzio and Miles 2014). More energy spent on territorial defence could translate into a reduced ability to attract further mates or attend multiple clutches, directly influencing mating systems. This conflict between aggression and direct care of offspring has been found in multiple animals (e.g. Lissåker and Kvarnemo 2006; DeAngelis et al. 2020). Importantly, filial cannibalism occurs in some dendrobatid frogs, both in adult males when taking over a new territory (e.g. Allobates femoralis: Ringler et al. 2017) and in females to decrease parental investment of a mate in unrelated clutches (e.g. Dendrobates auratus: Summers 1989). Thus, we predict that higher densities and lower resource availability could also lead to more territorial intrusions by males, more competition among females, and, as a result, an increase in filial cannibalism events.
Finally, because egg attendance and territorial defence may become more energetically demanding under harsh environmental conditions, we hypothesise that alternative care strategies such as plastic biparental care and monogamy could become favoured over evolutionary time. This is the case in the Atlantic labrid fish Symphodus tinca, which changes from no parental care to uniparental care when temperature and predators increase during the breeding season (Van den Berghe 1990), or in plovers (Charadrius spp.), where temperature stochasticity increased male parental cooperation during incubation (Vincze et al. 2017). Given that some poison frogs can show parental flexibility and a parent can take over tadpole transport when the other parent goes missing (for more details see next section), we encourage future studies to investigate whether flexibility can be found in other parental care behaviours such as egg attendance.
Consequences on larval survival and possible evolutionary trajectories under HIREC
The alteration of forest habitats for different human land-uses as well as changes in climate patterns can also affect poison frogs during larval and adult stages by modifying the availability and quality of important resources and microhabitats. For example, by clearing primary forest and reducing the canopy cover, the ground becomes more exposed to solar radiation, which increases near-ground temperature and, in turn, phytotelmata desiccation risk (del Pliego et al. 2016; Rivera-Ordonez et al. 2019). This is especially concerning given that the depletion of some resources (e.g. bromeliad phytotelmata) has been related to serious population declines in some poison frog species (Pröhl 2002; Vargas-Salinas and Amézquita 2013; Meza-Joya et al. 2015).
Phytotelmata are used in multiple poison frog species to deposit their tadpoles (Weygoldt 1987; Summers and McKeon 2004; Lehtinen et al. 2004; Rojas 2014, 2015; Schulte et al. 2020; Fouilloux et al. 2021), can naturally vary in water volume, nutrient composition, food sources, stability as well as the risk of competition and predation (Lehtinen et al. 2004). Consequently, parents have to assess all these different ecological factors, which can be highly unstable and vary in space and time (Rudolf and Rödel 2005; Schulte and Lötters 2013), and adapt their deposition strategy according to this information (Webb et al. 1999; Schulte and Lötters 2013). Furthermore, the size of these breeding pools has been associated with the evolution of different parental care strategies (e.g. trophic egg feeding and biparental care evolved in species using smaller pools; Brown et al. 2010). The selection of suitable rearing sites will play a key role in the successful development and survival of their offspring (Refsnider and Janzen 2010), and thus will have direct effects on the population recruitment for multiple species. However, HIREC might further exacerbate the instability and availability of good-quality phytotelmata, imposing multiple novel costs on parental care and territoriality. Because these stressors could be especially pronounced in small phytotelmata, species with parental care strategies such as egg feeding could be particularly affected. Importantly, nursery desiccation is already considered one of the most common abiotic causes of tadpole mortality (Fig. 1E), even in tropical rainforests where annual rainfall is very high (Murphy 2003; Rudolf and Rödel 2005; BR, CF pers. observ.).
Some authors have suggested plastic feeding behaviour as one possible mechanism to deal with phytotelmata desiccation. According to this hypothesis, some poison frog species would switch from avoiding tadpole/egg deposition in pools already containing conspecifics (to minimise predation: Caldwell and Araújo 1998; Summers 1999) to systematically depositing them with conspecifics, which can be a form of food resource. For example, in Ranitomeya ventrimaculata, clutches are laid more often in bromeliad axils where there is already a tadpole towards the end of the rainy season (Fig. 1F) (Poelman and Dicke 2007). This way, parents are thought to accelerate their older offspring’s development and increase their chance to reach metamorphosis before temporary pools dry out, which can happen within days. Likewise, older tadpoles of the species Ranitomeya variabilis may feed on younger siblings when resources are low (Brown et al. 2009). However, although cannibalising conspecific tadpoles provide higher nutritional value than other prey for some amphibian species (e.g. Crump 1990), the direct benefits of cannibalism through enhanced growth rates in poison frogs have not been disentangled from the benefits of eating ‘just’ another (i.e. heterospecific) tadpole. Instead, tadpole cannibalism is thought to be the result of indiscriminate predatory behaviour to eliminate potential competitors (Caldwell and Araújo 1998; Summers and McKeon 2004). Furthermore, weaker avoidance or even active choice of pools with conspecific tadpoles at the end of the rainy season could also be the result of less suitable sites available or parents using tadpole presence as a cue for pool quality and persistence, as is the case in Dendrobates tinctorius (Rojas 2014). This last idea is further supported by a study on Edalorhina perezi (Leptodactylidae), which also loses their sensitivity to invertebrate predators late in the rainy season (Murphy 2003).
A reduction in the number of suitable nurseries could also lead to the convergence of site choice by multiple parents, potentially from multiple species that in “normal” conditions would select for smaller/more unstable pool types (e.g. bromeliads). Consequently, we predict that under HIREC the overall larval density in pools will increase, and competition between tadpoles from the same or different species could become stronger, potentially benefitting certain species over others by exploiting alternative food supplies (i.e. feeding on other tadpoles of either the same (cannibalism) or different species). Cannibalism can have major consequences at the population level for some species, eliminating large proportions of offspring or entire cohorts in extreme cases (Polis 1981). That is the case in Ranitomeya (formerly Dendrobates) ventrimaculata, where only one tadpole survives in most pools regardless of the number of tadpoles deposited therein (Summers 1999).
We hypothesise that a reduction in the number of suitable phytotelmata available in a territory will force parents to transport their tadpoles longer distances until deposition sites, increasing direct and indirect associated costs. For example, transporting individuals might directly increase their mortality risk by presumably spending more time exposed to potential predators (Rojas and Endler 2013; Pašukonis et al. 2019), as well as indirectly reduce their fitness by investing less time and energy on territorial defence and mating opportunities (Pašukonis et al. 2019). From the larvae point of view, in dendrobatid species where adults transport tadpoles singly into phytotelmata, travelling longer distances would mean leaving siblings unattended for longer periods of time and, thus, increasing their probability of dying from desiccation, predation or fungal infection. All these costs may, in turn, become accentuated in human-disturbed habitats, where different microclimatic conditions, vegetation cover, and assemblages of predators pose new threats and increased stress (Knowlton and Graham 2010). One possible behavioural response that might be favoured to reduce the costs of transporting tadpoles longer distances could be to transport as many tadpoles as possible at the same time. Ringler et al. (2013) found a significantly positive correlation between the distance of Allobates femoralis males to their home territories during tadpole transport and the number of tadpoles on their back, suggesting that the number of tadpoles that parents decide to take up at once is influenced by the distance to suitable water bodies. This would mean that at least some species of poison frogs may be capable of adjusting their behaviour depending on the availability of tadpole deposition sites and buffer to some degree their reduction due to HIREC. Another response to deal with increased parental costs (i.e. longer transporting distances) that could be favoured over evolutionary time is the appearance of female parental care plasticity in otherwise uniparental male care systems. Because most female poison frogs do not defend territories (Pröhl 2005), they might gain considerable fitness benefits by flexibly taking over parental duties and increase the survival chances of the clutches in which they have already invested significant time and energy. Female parental care plasticity has been previously reported in some poison frogs (e.g. Allobates femoralis, Dendrobates tinctorius, Anomaloglossus beebei) where, in absence of the male caregiver, females show compensatory parental care behaviour by transporting tadpoles both under laboratory (Ringler et al. 2015; Fischer and O’Connell 2020) and natural conditions (Ringler et al. 2013; Rojas and Pašukonis 2019; Pettitt 2012). However, this plasticity has not been found in other close species like Allobates paleovarzensis (Rocha et al. 2021).
Finally, human-transformed habitats may also affect parents' orientation capacity by attenuating their familiarity with sensory cues. For example in Oophaga pumilio, orientation depends both on the distance and the habitat type (forests or pastures) (Nowakowski et al. 2013). Thus, given that males often select tadpole deposition sites outside of their territories or core areas (Ringler et al. 2013; Pašukonis et al. 2019), parents’ ability to find good rearing sites in the first place, or to return to selected phytotelmata in the case of tadpole feeding species, could be impaired. To date, very little work has explored the manner(s) in which land-use changes influence movement behaviour in poison frogs. However, it is reasonable to predict that they could have great impacts not only on parental decisions and territorial defence but also on population dispersal and gene flow. This is, therefore, a subject that merits further investigation.
Pet trade, infectious diseases, and pollution
In the Amazonian and Chocó rainforests, the fast development of large- and small-scale agriculture, urbanisation, and mining activities (Fig. 2), especially gold mining (Kalamandeen et al. 2018; Palacios-Torres et al. 2018), are not only modifying habitats but also polluting the environment (Folchi 2001; Piscoya Arbañil 2012; Gamarra Torres et al. 2018). Furthermore, accidental or deliberate introduction of exotic species, and, especially the global pet trade in the case of poison frogs, are increasing the transmission of and susceptibility to pathogens and parasites in previously isolated populations (e.g. Fecchio et al. 2021; Santos et al. 2021).
Illegal mining. Small-scale deforestation due to illegal mining activities is threatening the habitat of many species of poison frogs in the Amazon and the Chocó regions, two of Earth’s biodiversity hotspots. Here, illegal mining activity in Nouragues Natural Reserve, French Guiana. Photos: A) Bernard Gissinger; B) Alexandre David
The illegal pet trade is recognised as one of the major threats to dendrobatid poison frogs (Gorzula 1996; Gaucher and MacCulloch 2010; Nijman and Shepherd 2010; Brown et al. 2011; Betancourth-Cundar et al. 2020), as hobbyists are often after exotic colour variants, which can reach exorbitant prices in the market. This practice has been notably increasing in South America with the popularisation of the internet (Máximo et al. 2021), placing increased risks to the anurans of this region. Besides obvious long-term consequences such as decreased genetic diversity, the rarefaction of individuals in natural populations is thought to affect the two sexes differently, with males being at a higher risk of being detected due to the conspicuousness of their vocalisations (Fig. 1H) (Betancourth-Cundar et al. 2020), which they use to fend rivals off and to attract females. This can obviously alter the care provided to offspring, particularly in species in which parental care duties are predominantly performed by males, but it can also result in population declines as the populations end up being heavily female-biased (Betancourth-Cundar et al. 2020). The global amphibian pet trade is also widely recognised as one of the main drivers of the worldwide spread of amphibian pathogens such as the chytrid fungus Batrachochytrium dendrobatidis (hereafter Bd) (Fisher and Garner 2007), one of the most dramatic examples of newly-emerged pathogens, which causes the infectious disease chytridiomycosis. Therefore, it is not surprising that Bd has recently been detected in dendrobatid species in the wild.
Bd is known to be responsible for the mass mortalities in many amphibian populations and some species extinctions worldwide (Daszak et al. 2003; Lips et al. 2005; Pounds et al. 2006). Indeed, Bd prevalence in Dendrobatidae was recently found to be higher than in Bufonidae and Hylidae in an Amazonian community (Courtois et al. 2015). While the impact of Bd on poison frog populations is still poorly known, in other species it can inhibit the immune response (Fites et al. 2013), impact their body condition and growth (Parris and Cornelius 2004), reduce their locomotion and foraging performance (Chatfield et al. 2013; Venesky et al. 2009), and even change their advertisement calls (An and Waldman 2016). Moreover, because Bd zoospores are aquatic, species more dependent on water are expected to be the most impacted due to prolonged periods of time exposed to Bd zoospores (Bielby et al. 2008). Thus, in the scenario proposed above, where global change may cause higher densities of tadpoles sharing rearing sites, Bd transmission within and between species could exponentially increase. Likewise, we predict energetically costly activities such as parental care and territory defence to be also affected, because infected individuals may have to relocate energy from reproduction, calling, or parental care into immune defence. This means that infected individuals may be less able to defend their territories or perform parental care, which would indirectly cause higher offspring mortality rates. Given the importance of social behaviours on population dynamics, further research investigating the impacts of Bd on such behaviours is required.
In addition, chemical pollutants derived from agriculture (e.g. herbicides and pesticides) and mining activities (e.g. metals and metalloids: Hg, Cu, Co, Zn, and As) can impair individuals’ immune defences and further increase their susceptibility to pathogens and diseases (Christin et al. 2003). Similarly, when found in low concentrations, they can delay growth and metamorphosis (Carey and Bryant 1995), cause malformations (Unrine et al. 2004; Ferrante and Fearnside 2020), alter fertility and fecundity (Adams et al. 2021), or even cause sex-reversals (Nemesházi et al. 2020), often leading to devastating consequences for amphibian populations (Brühl et al. 2013). Increasing evidence demonstrates effects on a wide range of amphibian behaviours, such as reduced rates of activity (e.g. swimming, feeding, and breeding) or ability of tadpoles to escape predation (Shuman-Goodier and Propper 2016; Sievers et al. 2019). In two-lined salamanders (Eurycea bislineata), for instance, exposure to sublethal concentrations of mercury reduced their motivation to feed (Burke et al. 2010), whereas it impaired swimming performance in American toad (Anaxyrus [formerly Bufo] americanus) larvae (Bergeron et al. 2011). Although chemical contaminants have also been reported to alter multiple social behaviours such as territorial behaviour in other taxa (e.g. vom Saal et al. 1995; Bell 2001), to our knowledge, no study has addressed this possibility in amphibians. Given the strong detrimental effects of pollutants on egg and tadpole survival and development, we would expect selection to favour individuals capable of recognising and avoiding egg-laying and rearing sites based on chemical pollutant concentrations. As far as we are aware, however, this ability has been investigated in some anurans but not in poison frogs. For example, adults of the grey treefrog (Hyla versicolor) avoided ponds for oviposition if contaminated with the glyphosate pesticide Roundup (Takahashi 2007).
Social behaviours can also be impacted by an important, yet often underestimated, the form of anthropogenically driven pollution, noise pollution. For acoustically communicating species, as is the case of most anuran species, anthropogenic background noise can mask vocalisations and thus disrupt key species-specific communication (Simmons and Narins 2018). For instance, masking of acoustic signals could inhibit males’ calling activity (Sun and Narins 2005), reduce females’ ability to localise male’s advertisement calls (Caldwell and Bee 2014), or change female’s mate choice, potentially selecting for less fit males (Barrass 1985) or males with lower quality of parental care (Pettitt et al. 2020). Masked male calls may not only attract fewer females but also make territorial calls less audible, affecting male territorial defence by reducing their ability to detect and discriminate against conspecific intruders, as shown in birds (Kleist et al. 2016). This, in turn, may translate into more conspecific intrusions, aggressive encounters, and increased filial cannibalism rates. To cope with anthropogenic noise, some species can modify their call characteristics to contrast acoustically with noise pollution. For example, Cauca poison frogs, Andinobates bombetes (Fig. 1I), vocalise in moments of low background noise and call less when noise is higher (Vargas-Salinas and Amézquita 2013; Jiménez-Vargas and Vargas-Salinas 2021), while Bloody Bay poison frogs (Mannophryne olmonae) increase higher frequency calls and decrease inter-pulse intervals (Clemmens 2014). However, because changes in calling characteristics could potentially be opposed to female mate preferences, future research should investigate if such responses could become maladaptive.
Conclusions
-
1.
HIREC have great impacts on the way organisms interact among them and with their environment, imposing new threats for multiple species. Behaviour is often the first response to environmental changes, and its plasticity can determine how organisms adapt (or not) to HIREC. Social behaviour responses, in particular, are of special importance given their role in population dynamics (i.e. reproductive success and offspring survival). Thus, by combining animal behaviour and conservation issues, we can improve our understanding and predictions of how susceptible different species and populations are to HIREC.
-
2.
Due to their diverse and complex social behaviours, as well as their occurrence in often degraded habitats, poison frogs are an interesting group to study the potential impacts of and social responses to HIREC (see Fig. 3 for a summary).
-
3.
To compensate for negative HIREC impacts, we predict individuals to increase parental care efforts by spending more time attending clutches and transporting tadpoles to further and fewer nursery sites. However, this increase in parental costs will only be sustained to the point that it does not outweigh individual fitness. Furthermore, we hypothesise higher species-specific aggression rates both in adults and tadpoles, as well as more frequent filial cannibalistic events due to limited resources/territories and anthropogenic noise. Finally, altered environmental conditions derived from small-scale deforestation (i.e. higher radiation, increased temperature, and changes in ambient light) or increased noise pollution may disrupt important conspecific communication processes by reducing the calling capacity of males or by modifying mate detectability, courtship, and choice.
-
4.
Here, we have examined the impact of different anthropogenic stressors on poison frogs individually. However, the reality is usually more complex, with multiple HIREC and natural stressors acting and interacting simultaneously in unexpected ways. All these factors make predictions harder to formulate.
Conceptual overview. The main driving forces of HIREC (A climate change, B habitat fragmentation, C chemical pollution, and D novel pathogens and diseases) interact across habitats implicating cascading effects on the social behaviours of amphibians. Throughout the tropics, these disturbances will impact a large diversity of species with consequences detectable at every life stage. (I) We predict that HIREC will particularly threaten juveniles and larvae, where less consistent rainfall and higher temperatures will limit the availability and diversity of larval nurseries and increase the desiccation probability of clutches. (II) In response to these threats, we hypothesise that parents will both increase care and the flexibility in deposition choices
References
Adams E, Leeb C, Brühl CA (2021) Pesticide exposure affects reproductive capacity of common toads (Bufo bufo) in a viticultural landscape. Ecotoxicology 30(2):213–223. https://doi.org/10.1007/s10646-020-02335-9
Allee WC, Park O, Emerson AE, Park T, Schmidt KP (1949) Principles of animal ecology
Alonso-Alvarez C, Velando A, Royle NJ, Smiseth P T, Kölliker M (2012) The evolution of parental care. The evolution of parental care. 1st ed Oxford (UK): Oxford University Press. https://doi.org/10.1002/ajhb.22473
An D, Waldman B (2016) Enhanced call effort in Japanese tree frogs infected by amphibian chytrid fungus. Biol Let 12(3):20160018. https://doi.org/10.1098/rsbl.2016.0018
Armstrong TA (2019) The influence of maternal care duration on offspring phenotypes in African cichlids (Doctoral dissertation, University of Glasgow). https://doi.org/10.5525/gla.thesis.76736
Arroyo B, Razin M (2006) Effect of human activities on bearded vulture behaviour and breeding success in the French Pyrenees. Biol Cons 128(2):276–284. https://doi.org/10.1016/j.biocon.2005.09.035
Barnett JB, Varela BJ, Jennings BJ, Lesbarrères D, Pruitt JN, Green DM (2021) Habitat disturbance alters color contrast and the detectability of cryptic and aposematic frogs. Behav Ecol 32(5):814–825. https://doi.org/10.1093/beheco/arab032
Barras AN (1985) The effects of highway traffic noise on the phonotactic and associated reproductive behavior of selected anurans. (Doctoral dissertation, Vanderbuilt University)
Bell AM (2001) Effects of an endocrine disrupter on courtship and aggressive behaviour of male three-spined stickleback. Gasterosteus Aculeatus Animal Behaviour 62(4):775–780. https://doi.org/10.1006/anbe.2001.1824
Bergeron CM, Hopkins WA, Todd BD, Hepner MJ, Unrine JM (2011) Interactive effects of maternal and dietary mercury exposure have latent and lethal consequences for amphibian larvae. Environ Sci Technol 45(8):3781–3787. https://doi.org/10.1021/es104210a
Betancourth-Cundar M, Palacios-Rodríguez P, Mejía-Vargas D, Paz A, Amézquita A (2020) Genetic differentiation and overexploitation history of the critically endangered Lehmann’s Poison Frog: Oophaga lehmanni. Conserv Genet 21(3):453–465. https://doi.org/10.1007/s10592-020-01262-w
Bielby J, Cooper N, Cunningham AA, Garner TWJ, Purvis A (2008) Predicting susceptibility to future declines in the world’s frogs. Conservation Letters 1(2):82–90. https://doi.org/10.1111/j.1755-263X.2008.00015.x
Bredy TW, Lee AW, Meaney MJ, Brown RE (2004) Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus). Horm Behav 46(1):30–38. https://doi.org/10.1016/j.yhbeh.2003.09.017
Brenes-Soto A, Dierenfeld ES (2014) Effect of dietary carotenoids on vitamin A status and skin pigmentation in false tomato frogs (Dyscophus guineti). Zoo Biol 33(6):544–552. https://doi.org/10.1002/zoo.21175
Brown JL, Morales V, Summers K (2009) Tactical reproductive parasitism via larval cannibalism in Peruvian poison frogs. Biol Let 5(2):148–151. https://doi.org/10.1098/rsbl.2008.0591
Brown JL, Morales V, Summers K (2010) A key ecological trait drove the evolution of biparental care and monogamy in an amphibian. Am Nat 175(4):436–446. https://doi.org/10.1086/650727
Brown JL, Twomey E, Amezquita A, De Souza MB, Caldwell JP et al (2011) A taxonomic revision of the Neotropical poison frog genus Ranitomeya (Amphibia: Dendrobatidae). Zootaxa 3083(1):1–120. https://doi.org/10.1055/sos-SD-201-00174
Brown JL, Twomey E, Morales V, Summers K (2008) Phytotelm size in relation to parental care and mating strategies in two species of Peruvian poison frogs. Behaviour 1139–1165. https://doi.org/10.1163/156853908785387647
Brühl CA, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure of amphibians: an underestimated cause of global decline? Sci Rep 3(1):1–4. https://doi.org/10.1038/srep01135
Burke JN, Bergeron CM, Todd BD, Hopkins WA (2010) Effects of mercury on behavior and performance of northern two-lined salamanders (Eurycea bislineata). Environ Pollut 158(12):3546–3551. https://doi.org/10.1016/j.envpol.2010.08.017
Bustnes JO, Erikstad KE (1991) Parental care in the common eider (Somateria mollissima): factors affecting abandonment and adoption of young. Can J Zool 69(6):1538–1545. https://doi.org/10.1139/z91-216
Candolin U, Wong BB (Eds.) (2012) Behavioural responses to a changing world: mechanisms and consequences. Oxford University Press
Caldwell JP, de Araújo MC (1998) Cannibalistic interactions resulting from indiscriminate predatory behavior in tadpoles of poison frogs (Anura: Dendrobatidae) 1. Biotropica 30(1):92–103. https://doi.org/10.1111/j.1744-7429.1998.tb00372.x
Caldwell MS, Bee MA (2014) Spatial hearing in Cope’s gray treefrog: I. Open and closed loop experiments on sound localization in the presence and absence of noise. J Comp Physiol A 200(4):265–284. https://doi.org/10.1007/s00359-014-0882-6
Carey C, Bryant CJ (1995) Possible interrelations among environmental toxicants, amphibian development, and decline of amphibian populations. Environ Health Perspect 103(suppl 4):13–17. https://doi.org/10.1289/ehp.103-1519280
Caro T (1999) The behaviour–conservation interface. Trends Ecol Evol 14(9):366–369. https://doi.org/10.1016/S0169-5347(99)01663-8
Carvajal-Castro JD, Vargas-Salinas F, Casas-Cardona S, Rojas B, Santos JC (2021) Aposematism facilitates the diversification of parental care strategies in poison frogs. Sci Rep 11(1):1–15. https://doi.org/10.1038/s41598-021-97206-6
Chatfield MW, Brannelly LA, Robak MJ, Freeborn L, Lailvaux SP, Richards-Zawacki CL (2013) Fitness consequences of infection by Batrachochytrium dendrobatidis in northern leopard frogs (Lithobates pipiens). EcoHealth 10(1):90–98. https://doi.org/10.1007/s10393-013-0833-7
Christin MS, Gendron AD, Brousseau P, Ménard L, Marcogliese DJ, Cyr D, Fournier M (2003) Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environ Toxicol Chem: Int J 22(5):1127–1133. https://doi.org/10.1002/etc.5620220522
Clemmens CG (2014) A not-so-silent spring: the impacts of traffic noise on call features of the bloody bay poison frog (Mannophryne olmonae)
Cope ED (1865) Sketch of the primary groups of Batrachia Salientia. Natural History Review, New Series 5:97–120
Cordier JM, Aguilar R, Lescano JN, Leynaud GC, Bonino A, Miloch D, Nori J (2021) A global assessment of amphibian and reptile responses to land-use changes. Biol Cons 253:108863. https://doi.org/10.1016/j.biocon.2020.108863
Courtois EA, Gaucher P, Chave J, Schmeller DS (2015) Widespread occurrence of Bd in French Guiana. South America Plos One 10(4):e0125128. https://doi.org/10.1371/journal.pone.0125128
Cowie RH, Bouchet P, Fontaine B (2022) The sixth mass extinction: fact, fiction or speculation? Biol Rev. https://doi.org/10.1111/brv.12816
Crispo E (2007) The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evol: Int J Organic Evol 61(11):2469–2479. https://doi.org/10.1111/j.1558-5646.2007.00203.x
Croft DP, James R, Krause J (2008) Exploring animal social networks. Princeton University Press. https://doi.org/10.1515/9781400837762
Crothers LR, Cummings ME (2015) A multifunctional warning signal behaves as an agonistic status signal in a poison frog. Behav Ecol 26:560–568. https://doi.org/10.1093/beheco/aru231
Crump ML (1990) Possible enhancement of growth in tadpoles through cannibalism. Copeia 1990(2):560–564. https://doi.org/10.2307/1446361
Daszak P, Cunningham AA, Hyatt AD (2003) Infectious disease and amphibian population declines. Divers Distrib 9(2):141–150. https://doi.org/10.1046/j.1472-4642.2003.00016.x
DeAngelis R, Dodd L, Rhodes J (2020) Nonapeptides mediate trade-offs in parental care strategy. Horm Behav 121:104717. https://doi.org/10.1016/j.yhbeh.2020.104717
Delia JR, Ramírez-Bautista A, Summers K (2013) Parents adjust care in response to weather conditions and egg dehydration in a Neotropical glassfrog. Behav Ecol Sociobiol 67(4):557–569. https://doi.org/10.1007/s00265-013-1475-z
Delia J, Bravo-Valencia L, Warkentin KM (2020) The evolution of extended parental care in glassfrogs: do egg-clutch phenotypes mediate coevolution between the sexes? Ecol Monogr 90(3):e01411. https://doi.org/10.1002/ecm.1411
del Pliego PG, Scheffers BR, Basham EW, Woodcock P, Wheeler C, Gilroy JJ, Edwards DP (2016) Thermally buffered microhabitats recovery in tropical secondary forests following land abandonment. Biol Cons 201:385–395. https://doi.org/10.1016/j.biocon.2016.07.038
Dick JT, Faloon SE, Elwood RW (1998) Active brood care in an amphipod: influences of embryonic development, temperature and oxygen. Anim Behav 56(3):663–672. https://doi.org/10.1006/anbe.1998.0797
Donnelly MA, Crump ML (1998) Potential effects of climate change on two neotropical amphibian assemblages. Clim Change 39(2):541–561. https://doi.org/10.1007/978-94-017-2730-3_20
Duellman WE, Trueb L (1994) Biology of amphibians. JHU Press
Dugas MB, Yeager J, Richards-Zawacki CL (2013) Carotenoid supplementation enhances reproductive success in captive strawberry poison frogs (Oophaga pumilio). Zoo Biol 32(6):655–658. https://doi.org/10.1002/zoo.21102
Endler JA (1993) The color of light in forests and its implications. Ecol Monogr 63(1):1–27. https://doi.org/10.2307/2937121
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34(1):487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Fecchio A, de Faria IP, Bell JA, Nunes R, Weckstein JD, Lima MR (2021) Mining increases the prevalence of avian haemosporidian parasites in Northeast Amazonia. Parasitol Res 120(2):605–613. https://doi.org/10.1007/s00436-020-06986-9
Ferrante L, Fearnside PM (2020) Evidence of mutagenic and lethal effects of herbicides on Amazonian frogs. Acta Amazon 50:363–366. https://doi.org/10.1590/1809-4392202000562
Fischer EK, O’Connell LA (2020) Hormonal and neural correlates of care in active versus observing poison frog parents. Horm Behav 120:104696. https://doi.org/10.1016/j.yhbeh.2020.104696
Fisher DN, Kilgour RJ, Siracusa ER, Foote JR, Hobson EA, Montiglio PO, Wice EW (2021) Anticipated effects of abiotic environmental change on intraspecific social interactions. Biol Rev 96(6):2661–2693. https://doi.org/10.1111/brv.12772
Fisher MC, Garner TW (2007) The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biol Rev 21(1):2–9. https://doi.org/10.1016/j.fbr.2007.02.002
Fites JS, Ramsey JP, Holden WM, Collier SP, Sutherland DM, Reinert LK, Rollins-Smith LA (2013) The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 342(6156):366–369. https://doi.org/10.1126/science.1243316
Folchi M (2001) Conflictos de contenido ambiental y ecologismo de los pobres: no siempre pobres, ni siempre ecologistas. Ecología Política 22:79–100
Fouilloux CA, Serrano Rojas SJ, Carvajal-Castro JD, Valkonen JK, Gaucher P, Fischer MT, Rojas B (2021) Pool choice in a vertical landscape: tadpole-rearing site flexibility in phytotelm-breeding frogs. Ecol Evol. https://doi.org/10.1002/ece3.7741
Gamarra Torres OA, Barrena Gurbillón MA, Barboza Castillo E, Rascón Barrios J, Corroto F, Taramona Ruiz LA (2018) Fuentes de contaminación estacionales en la cuenca del río Utcubamba, región Amazonas, Perú. Arnaldoa 25(1):179–194. http://www.scielo.org.pe/pdf/arnal/v25n1/a11v25n1.pdf
Gaucher P, MacCulloch R (2010) Dendrobates tinctorius. The IUCN Red List of Threatened Species 2010:e.T55204A11265402 https://doi.org/10.2305/IUCN.UK.2010-2.RLTS.T55204A11265402.en
Gorzula S (1996) The trade in dendrobatid frogs from 1987 to 1993. Herpetol Rev 27(3):116–122
Grant T, Frost DR, Caldwell JP, Gagliardo RON, Haddad CF, Kok PJ, Wheeler WC (2006) Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae). Bull Am Mus Nat Hist 2006(299):1–262. https://doi.org/10.1206/0003-0090(2006)299[1:PSODFA]2.0.CO;2
Grau HR, Aide M (2008) Globalization and land-use transitions in Latin America. Ecol Soc 13(2)
Green BS, McCormick MI (2005) O2 replenishment to fish nests: males adjust brood care to ambient conditions and brood development. Behav Ecol 16(2):389–397. https://doi.org/10.1093/beheco/ari007
Gromov VS (2009) Interactions of partners in family pairs, care of the offspring, and the role of tactile stimulation in formation of parental behavior of the Mongolian gerbil (Meriones unguiculatus) under laboratory conditions. Biology Bulletin 36(5):479–488. https://doi.org/10.1134/S1062359009050082
Hendry AP, Farrugia TJ, Kinnison MT (2008) Human influences on rates of phenotypic change in wild animal populations. Mol Ecol 17(1):20–29. https://doi.org/10.1111/j.1365-294X.2007.03428.x
Hopkins WA (2007) Amphibians as models for studying environmental change. ILAR J 48(3):270–277. https://doi.org/10.1093/ilar.48.3.270
Hugo G (2008) Trends in land degradation in South America. In Management of natural and environmental resources for sustainable agricultural development. World Meteorological Organization, Workshop Proceedings. Portland, Oregon
IUCN (2020) The IUCN red list of threatened species. International Union for Conservation of Nature and Natural Resources
Jiménez-Muñoz JC, Mattar C, Barichivich J, Santamaría-Artigas A, Takahashi K, Malhi Y, Van Der Schrier G (2016) Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015–2016. Sci Rep 6(1):1–7. https://doi.org/10.1038/srep33130
Jiménez-Vargas GM, Vargas-Salinas F (2021) Does anthropogenic noise promote advertisement call adjustments in the rubí poison frog Andinobates bombetes?. Behaviour 1(aop) 1–19. https://doi.org/10.1163/1568539X-bja10080
Kalamandeen M, Gloor E, Mitchard E, Quincey D, Ziv G, Spracklen D, Galbraith D (2018) Pervasive rise of small-scale deforestation in Amazonia. Sci Rep 8(1):1–10. https://doi.org/10.1038/s41598-018-19358-2
Kelly E, Phillips BL (2017) Get smart: native mammal develops toad-smart behavior in response to a toxic invader. Behav Ecol 28(3):854–858. https://doi.org/10.1093/beheco/arx045
Kleist NJ, Guralnick RP, Cruz A, Francis CD (2016) Anthropogenic noise weakens territorial response to intruder’s songs. Ecosphere 7(3):e01259. https://doi.org/10.1002/ecs2.1259
Knowlton JL, Graham CH (2010) Using behavioral landscape ecology to predict species’ responses to land-use and climate change. Biol Cons 143(6):1342–1354. https://doi.org/10.1016/j.biocon.2010.03.011
Kriska G, Horváth G, Andrikovics S (1998) Why do mayflies lay their eggs en masse on dry asphalt roads? Water-imitating polarized light reflected from asphalt attracts Ephemeroptera. J Exp Biol 201(15):2273–2286. https://doi.org/10.1242/jeb.201.15.2273
Kriska G, Malik P, Szivák I, Horváth G (2008) Glass buildings on river banks as “polarized light traps” for mass-swarming polarotactic caddis flies. Naturwissenschaften 95(5):461–467. https://doi.org/10.1007/s00114-008-0345-4
Lattanzio MS, Miles DB (2014) Ecological divergence among colour morphs mediated by changes in spatial network structure associated with disturbance. J Anim Ecol 83(6):1490–1500. https://doi.org/10.1111/1365-2656.12252
Lehtinen RM, Lannoo MJ, Wassersug RJ (2004) Phytotelm-breeding anurans: past, present and future research. Miscellaneous Publications, Museum of Zoology, University of Michigan 193:1–9
Lewis SL, Edwards DP, Galbraith D (2015) Increasing human dominance of tropical forests. Science 349(6250):827–832. https://doi.org/10.1126/science.aaa9932
Lips KR, Burrowes PA, Mendelson JR III, Parra‐Olea G (2005) Amphibian population declines in Latin America: a synthesis 1. Biotropica: The J Biol Conserv 37(2):222–226. https://doi.org/10.1111/j.1744-7429.2005.00029.x
Lissåker M, Kvarnemo C (2006) Ventilation or nest defense—parental care trade-offs in a fish with male care. Behav Ecol Sociobiol 60(6):864–873. https://doi.org/10.1007/s00265-006-0230-0
Liu D, Diorio J, Day JC, Francis DD, Meaney MJ (2000) Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci 3(8):799–806. https://doi.org/10.1038/77702
Lockwood JL, Hoopes MF, Marchetti MP (2013) Invasion ecology. John Wiley & Sons
Lovejoy TE, Nobre C (2018) Amazon tipping point. Sci Adv 4(2):eaat2340. https://doi.org/10.1126/sciadv.aat2340
Maan ME, Cummings ME (2008) Female preferences for aposematic signal components in a polymorphic poison frog. Evol: Int J Organic Evol 62(9):2334–2345. https://doi.org/10.1111/j.1558-5646.2008.00454.x
Máximo IM, Brandao RA, Ruggeri J, Toledo LF (2021) Amphibian illegal pet trade and a possible new case of an invasive exotic species in Brazil. Herpetol Conserv Biol 16(2):303–312. https://doi.org/10.1186/1471-2156-14-70
Meza-Joya FL, Ramos-Pallares E, Hernández-Jaimes C (2015) Use of an agroecosystem by the threatened dart poison frog Andinobates virolinensis (Dendrobatidae). Herpetol Rev 46(2):171–176
Mullu, D. (2016). A review on the effect of habitat fragmentation on ecosystem. Journal of Natural Sciences Research, 6(15), 1–15.Murphy, P. J (2003) Does reproductive site choice in a neotropical frog mirror variable risks facing offspring? Ecol Monogr 73(1):45–67. https://doi.org/10.1890/0012-9615(2003)073[0045:DRSCIA]2.0.CO;2
Murphy PJ (2003) Context-dependent reproductive site choice in a Neotropical frog. Behav Ecol 14(5):626–633. https://doi.org/10.1093/beheco/arg042
Nemesházi E, Gál Z, Ujhegyi N, Verebélyi V, Mikó Z, Üveges B, Bókony V (2020) Novel genetic sex markers reveal high frequency of sex reversal in wild populations of the agile frog (Rana dalmatina) associated with anthropogenic land use. Mol Ecol 29(19):3607–3621. https://doi.org/10.1111/mec.15596
Nijman V, Shepherd CR (2010) The role of Asia in the global trade in CITES II-listed poison arrow frogs: hopping from Kazakhstan to Lebanon to Thailand and beyond. Biodivers Conserv 19(7):1963–1970. https://doi.org/10.1007/s10531-010-9814-0
Nori J, Lemes P, Urbina-Cardona N, Baldo D, Lescano J, Loyola R (2015) Amphibian conservation, land-use changes and protected areas: a global overview. Biol Cons 191:367–374. https://doi.org/10.1016/j.biocon.2015.07.028
Nowakowski AJ, Otero Jiménez B, Allen M, Diaz-Escobar M, Donnelly MA (2013) Landscape resistance to movement of the poison frog, Oophaga pumilio, in the lowlands of northeastern Costa Rica. Anim Conserv 16(2):188–197. https://doi.org/10.1111/j.1469-1795.2012.00585.x
Nowicki S, Searcy WA, Peters S (2002) Brain development, song learning and mate choice in birds: a review and experimental test of the “nutritional stress hypothesis.” J Comp Physiol A 188(11):1003–1014. https://doi.org/10.1007/s00359-002-0361-3
Öberg M, Arlt D, Pärt T, Laugen AT, Eggers S, Low M (2015) Rainfall during parental care reduces reproductive and survival components of fitness in a passerine bird. Ecol Evol 5(2):345–356. https://doi.org/10.1002/ece3.1345
Ogilvy V, Preziosi RF, Fidgett AL (2012) A brighter future for frogs? The influence of carotenoids on the health, development and reproductive success of the red-eye tree frog. Anim Conserv 15(5):480–488. https://doi.org/10.1111/j.1469-1795.2012.00536.x
Palacios-Torres Y, Caballero-Gallardo K, Olivero-Verbel J (2018) Mercury pollution by gold mining in a global biodiversity hotspot, the Choco biogeographic region, Colombia. Chemosphere 193:421–430. https://doi.org/10.1016/j.chemosphere.2017.10.160
Palumbi SR (2001) Humans as the World’s Greatest Evolutionary Force. Science 293(5536):1786–1790. https://doi.org/10.1126/science.293.5536.1786
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421(6918):37–42. https://doi.org/10.1038/nature01286
Parris MJ, Cornelius TO (2004) Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology 85(12):3385–3395. https://doi.org/10.1890/04-0383
Pašukonis A, Loretto MC, Rojas B (2019) How far do tadpoles travel in the rainforest? Parent-assisted dispersal in poison frogs. Evol Ecol 33(4):613–623. https://doi.org/10.1007/s10682-019-09994-z
Pettitt BA (2012) Paternal effort in relation to acoustically mediated mate choice in a Neotropical frog. University of Minnesota
Pettitt BA, Bourne GR, Bee MA (2020) Females prefer the calls of better fathers in a Neotropical frog with biparental care. Behav Ecol 31(1):152–163. https://doi.org/10.1093/beheco/arz172
Pigliucci M (2001) Phenotypic plasticity: beyond nature and nurture. JHU Press
Pimm SL, Raven P (2000) Extinction by numbers. Nature 403(6772):843–845. https://doi.org/10.1038/35002708
Piscoya Arbañil JA (2012) Minería y contaminación ambiental en Piura. https://doi.org/10.33017/RevECIPeru2011.0053
Poelman EH, Dicke M (2007) Offering offspring as food to cannibals: oviposition strategies of Amazonian poison frogs (Dendrobates ventrimaculatus). Evol Ecol 21(2):215–227. https://doi.org/10.1007/s10682-006-9000-8
Polis GA (1981) The evolution and dynamics of intraspecific predation. Annu Rev Ecol Syst 12(1):225–251. https://doi.org/10.1146/annurev.es.12.110181.001301
Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MP, Foster PN, Young BE (2006) Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439(7073):161–167. https://doi.org/10.1038/nature04246
Pröhl H (2002) Population differences in female resource abundance, adult sex ratio, and male mating success in Dendrobates pumilio. Behav Ecol 13(2):175–181. https://doi.org/10.1093/beheco/13.2.175
Pröhl H (2005) Territorial behavior in dendrobatid frogs. J Herpetol 39(3):354–365. https://doi.org/10.1670/162-04A.1
Pröhl H, Ostrowski T (2011) Behavioural elements reflect phenotypic colour divergence in a poison frog. Evol Biol 25:993–1015. https://doi.org/10.1007/s10682-010-9455-5
Ratikainen II, Haaland TR, Wright J (2018) Differential allocation of parental investment and the trade-off between size and number of offspring. Proc R Soc B 285(1884):20181074. https://doi.org/10.1098/rspb.2018.1074
Refsnider JM, Janzen FJ (2010) Putting eggs in one basket: ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu Rev Ecol Evol Syst 41:39–57. https://doi.org/10.1146/annurev-ecolsys-102209-144712
Relyea RA, Mills N (2001) Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor). Proc Natl Acad Sci 98(5):2491–2496. https://doi.org/10.1073/pnas.031076198
Ricklefs RE, Schluter D (Eds.) (1993) Species diversity in ecological communities: historical and geographical perspectives (Vol. 7). Chicago: University of Chicago Press. https://doi.org/10.1046/j.1420-9101.1994.7050635.x
Ringler E, Pašukonis A, Hödl W, Ringler M (2013) Tadpole transport logistics in a Neotropical poison frog: indications for strategic planning and adaptive plasticity in anuran parental care. Front Zool 10(1):1–10. https://doi.org/10.1186/1742-9994-10-67
Ringler E, Pašukonis A, Fitch WT, Huber L, Hödl W, Ringler M (2015) Flexible compensation of uniparental care: female poison frogs take over when males disappear. Behav Ecol 26(4):1219–1225. https://doi.org/10.1093/beheco/arv069
Ringler E, Beck KB, Weinlein S, Huber L, Ringler M (2017) Adopt, ignore, or kill? Male poison frogs adjust parental decisions according to their territorial status. Sci Rep 7(1):1–6. https://doi.org/10.1038/srep43544
Rivera-Ordonez JM, Justin Nowakowski A, Manansala A, Thompson ME, Todd BD (2019) Thermal niche variation among individuals of the poison frog, Oophaga pumilio, in forest and converted habitats. Biotropica 51(5):747–756. https://doi.org/10.1111/btp.12691
Robertson BA, Rehage JS, Sih A (2013) Ecological novelty and the emergence of evolutionary traps. Trends Ecol Evol 28(9):552–560. https://doi.org/10.1016/j.tree.2013.04.004
Rocha S, Lima AP, Kaefer IL (2021) Key roles of paternal care and climate on offspring survival of an Amazonian poison frog. An Acad Bras Ciênc 93. https://doi.org/10.1590/0001-376520212021006
Rohr JR, Kerby JL, Sih A (2006) Community ecology as a framework for predicting contaminant effects. Trends Ecol Evol 21(11):606–613. https://doi.org/10.1016/j.tree.2006.07.002
Rojas B (2014) Strange parental decisions: fathers of the dyeing poison frog deposit their tadpoles in pools occupied by large cannibals. Behav Ecol Sociobiol 68(4):551–559. https://doi.org/10.1007/s00265-013-1670-y
Rojas B (2015) Mind the gap: treefalls as drivers of parental trade-offs. Ecol Evol 5(18):4028–4036. https://doi.org/10.1002/ece3.1648
Rojas B, Endler JA (2013) Sexual dimorphism and intra-populational colour pattern variation in the aposematic frog Dendrobates tinctorius. Evol Ecol 27(4):739–753. https://doi.org/10.1007/s10682-013-9640-4
Rojas B, Pašukonis A (2019) From habitat use to social behavior: natural history of a voiceless poison frog. Dendrobates Tinctorius Peerj 7:e7648. https://doi.org/10.7717/peerj.7648
Rojas B, Rautiala P, Mappes J (2014) Differential detectability of polymorphic warning signals under varying light environments. Behav Proc 109:164–172. https://doi.org/10.1016/j.beproc.2014.08.014
Rosenzweig ML (1995) Species Diversity in Space and Time. Cambridge University Press, New York. http://dx.doi.org/10.1017/CBO9780511623387
Royle NJ, Smiseth PT, Kölliker M (2012) The evolution of parental care. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:oso/9780199692576.001.0001
Rudh A, Breed MF, Qvarnström A (2013) Does aggression and explorative behaviour decrease with lost warning coloration? Biol J Linn Soc 108(1):116–126. https://doi.org/10.1111/j.1095-8312.2012.02006.x
Rudolf VH, Rödel MO (2005) Oviposition site selection in a complex and variable environment: the role of habitat quality and conspecific cues. Oecologia 142(2):316–325. https://doi.org/10.1007/s00442-004-1668-2
Santos WS, Gurgel-Gonçalves R, Garcez LM, Abad-Franch F (2021) Deforestation effects on Attalea palms and their resident Rhodnius, vectors of Chagas disease, in eastern Amazonia. PLoS ONE 16(5):e0252071. https://doi.org/10.1371/journal.pone.0252071
Schroeder J, Nakagawa S, Hinsch M (2011) Behavioural ecology is not an endangered discipline. Trends Ecol Evol 26(7):320–321. https://doi.org/10.1016/j.tree.2011.03.013
Schulte LM, Lötters S (2013) The power of the seasons: rainfall triggers parental care in poison frogs. Evol Ecol 27(4):711–723. https://doi.org/10.1007/s10682-013-9637-z
Schulte LM, Ringler E, Rojas B, Stynoski JL (2020) Developments in amphibian parental care research: history, present advances, and future perspectives. Herpetol Monogr 34(1):71–97. https://doi.org/10.1655/HERPMONOGRAPHS-D-19-00002.1
Shuman-Goodier ME, Propper CR (2016) A meta-analysis synthesizing the effects of pesticides on swim speed and activity of aquatic vertebrates. Sci Total Environ 565:758–766. https://doi.org/10.1016/j.scitotenv.2016.04.205
Sievers M, Hale R, Parris KM, Melvin SD, Lanctot CM, Swearer SE (2019) Contaminant-induced behavioural changes in amphibians: a meta-analysis. Sci Total Environ 693:133570. https://doi.org/10.1016/j.scitotenv.2019.07.376
Sih A (2013) Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim Behav 85(5):1077–1088. https://doi.org/10.1016/j.anbehav.2013.02.017
Sih A, Ferrari MC, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4(2):367–387. https://doi.org/10.1111/j.1752-4571.2010.00166.x
Simmons AM, Narins PM (2018) Effects of anthropogenic noise on amphibians and reptiles. In Effects of anthropogenic noise on animals (pp. 179–208). Springer, New York, NY. https://doi.org/10.1007/978-1-4939-8574-6_7
Slabbekoorn H, Peet M (2003) Birds sing at a higher pitch in urban noise. Nature 424(6946):267–267. https://doi.org/10.1038/424267a
Souza JR, Kaefer IL, Lima AP (2017) The peculiar breeding biology of the Amazonian frog Allobates subfolionidificans (Aromobatidae). An Acad Bras Ciênc 89:885–893. https://doi.org/10.1590/0001-37652017201602
Stahlschmidt Z, DeNardo DF (2010) Parental behavior in pythons is responsive to both the hydric and thermal dynamics of the nest. J Exp Biol 213(10):1691–1696. https://doi.org/10.1242/jeb.041095
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306(5702):1783–1786. https://doi.org/10.1126/science.1103538
Stückler S, Cloer S, Hödl W, Preininger D (2022) Carotenoid intake during early life mediates ontogenetic colour shifts and dynamic colour change during adulthood. Anim Behav 187:121–135. https://doi.org/10.1016/j.anbehav.2022.03.007
Summers K (1989) Sexual selection and intra-female competition in the green poison-dart frog, Dendrobates auratus. Anim Behav 37:797–805. https://doi.org/10.1016/0003-3472(89)90064-X
Summers K (1999) The effects of cannibalism on Amazonian poison frog egg and tadpole deposition and survivorship in Heliconia axil pools. Oecologia 119(4):557–564. https://doi.org/10.1007/s004420050819
Summers K, Weigt LA, Boag P, Bermingham E (1999a) The evolution of female parental care in poison frogs of the genus Dendrobates: evidence from mitochondrial DNA sequences. Herpetologica 254–270
Summers K, Symula R, Clough M, Cronin T (1999b) Visual mate choice in poison frogs. Proceedings of the Royal Society of London. Series B: Biol Sci 266(1434):2141–2145. https://doi.org/10.1098/rspb.1999.0900
Summers K, McKeon CS (2004) The evolutionary ecology of phytotelmata use in Neotropical poison frogs. Miscellaneous Publications, Museum of Zoology, University of Michigan 193:55–73
Summers K, Tumulty J (2014) Parental care, sexual selection, and mating systems in neotropical poison frogs. Sexual Select (pp. 289–320). Academic Press. https://doi.org/10.1016/B978-0-12-416028-6.00011-6
Sun JW, Narins PM (2005) Anthropogenic sounds differentially affect amphibian call rate. Biol Cons 121(3):419–427. https://doi.org/10.1016/j.biocon.2004.05.017
Suski CD, Ridgway MS (2007) Climate and body size influence nest survival in a fish with parental care. J Anim Ecol 730–739. https://doi.org/10.1111/j.1365-2656.2007.01242.x
Takahashi M (2007) Oviposition site selection: pesticide avoidance by gray treefrogs. Environ Toxicol Chem: Int J 26(7):1476–1480. https://doi.org/10.1897/06-511R.1
Taubert F, Fischer R, Groeneveld J, Lehmann S, Müller MS et al (2018) Global patterns of tropical forest fragmentation. Nature 554(7693):519–522. https://doi.org/10.1038/nature25508
Théry M, Endler JA (2001) Habitat selection, ambient light and colour patterns in some lek-displaying birds. In Nouragues (pp. 161–166). Springer, Dordrecht. https://doi.org/10.1007/978-94-015-9821-7_14
Touchon JC (2012) A treefrog with reproductive mode plasticity reveals a changing balance of selection for nonaquatic egg laying. Am Nat 180(6):733–743. https://doi.org/10.1086/668079
Touchon JC, Warkentin KM (2009) Negative synergism of rainfall patterns and predators affects frog egg survival. J Anim Ecol 78(4):715–723. https://doi.org/10.1111/j.1365-2656.2009.01548.x
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86(3):640–657. https://doi.org/10.1111/j.1469-185X.2010.00164.x
Tumulty J, Morales V, Summers K (2014) The biparental care hypothesis for the evolution of monogamy: experimental evidence in an amphibian. Behav Ecol 25(2):262–270. https://doi.org/10.1093/beheco/art116
Tuxbury SM, Salmon M (2005) Competitive interactions between artificial lighting and natural cues during seafinding by hatchling marine turtles. Biol Cons 121(2):311–316. https://doi.org/10.1016/j.biocon.2004.04.022
Twomey E, Johnson JD, Castroviejo-Fisher S, Van Bocxlaer I (2020) A ketocarotenoid-based colour polymorphism in the Sira poison frog Ranitomeya sirensis indicates novel gene interactions underlying aposematic signal variation. Mol Ecol 29(11):2004–2015. https://doi.org/10.1111/mec.15466
Umbers KD, Silla AJ, Bailey JA, Shaw AK, Byrne PG (2016) Dietary carotenoids change the colour of Southern corroboree frogs. Biol J Lin Soc 119(2):436–444. https://doi.org/10.1111/bij.12818
Unrine JM, Jagoe CH, Hopkins WA, Brant HA (2004) Adverse effects of ecologically relevant dietary mercury exposure in southern leopard frog (Rana sphenocephala) larvae. Environ Toxicol Chem: Int J 23(12):2964–2970. https://doi.org/10.1897/03-696.1
Van den Berghe EP (1990) Parental care evolution in a labrid fish: Symphodus tinca
Vargas-Salinas F, Amézquita A (2013) Traffic noise correlates with calling time but not spatial distribution in the threatened poison frog Andinobates bombetes. Behaviour 150(6):569–584. https://doi.org/10.1163/1568539X-00003068
Vasconcelos RO, Amorim MCP, Ladich F (2007) Effects of ship noise on the detectability of communication signals in the Lusitanian toadfish. J Exp Biol 210(12):2104–2112. https://doi.org/10.1242/jeb.004317
Venesky MD, Parris MJ, Storfer A (2009) Impacts of Batrachochytrium dendrobatidis infection on tadpole foraging performance. EcoHealth 6(4):565–575. https://doi.org/10.1007/s10393-009-0272-7
Vincze O, Kosztolányi A, Barta Z, Küpper C, Alrashidi M, Amat JA, Székely T (2017) Parental cooperation in a changing climate: fluctuating environments predict shifts in care division. Glob Ecol Biogeogr 26(3):347–358. https://doi.org/10.1111/geb.12540
Vitt LJ, Avila-Pires TC, Caldwell JP, Oliveira VR (1998) The impact of individual tree harvesting on thermal environments of lizards in Amazonian rain forest. Conserv Biol 12(3):654–664. https://doi.org/10.1111/j.1523-1739.1998.96407.x
Vom Saal FS, Nagel SC, Palanza P, Boechler M, Parmigiani S, Welshons WV (1995) Estrogenic pesticides: binding relative to estradiol in MCF-7 cells and effects of exposure during fetal life on subsequent territorial behaviour in male mice. Toxicol Lett 77(1–3):343–350. https://doi.org/10.1016/0378-4274(95)03316-5
Wake DB, Vredenburg VT (2008) Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci 105(Supplement 1):11466–11473. https://doi.org/10.1073/pnas.0801921105
Webb JN, Houston AI, McNamara JM, SzéKely T (1999) Multiple patterns of parental care. Anim Behav 58:983–993. https://doi.org/10.1006/anbe.1999.1215
Wells KD (2010) The ecology and behavior of amphibians. University of Chicago Press. https://doi.org/10.7208/9780226893334
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press
Weygoldt P (1987) Evolution of parental care in dart poison frogs (Amphibia: Anura: Dendrobatidae). J Zool Syst Evol Res 25(1):51–67. https://doi.org/10.1111/j.1439-0469.1987.tb00913.x
Willink B, Brenes-Mora E, Bolaños F, Pröhl H (2013) Not everything is black and white: color and behavioral variation reveal a continuum between cryptic and aposematic strategies in a polymorphic poison frog. Evolution 67(10):2783–2794. https://doi.org/10.1111/evo.12153
Willink B, Bolaños F, Pröhl H (2014) Conspicuous displays in cryptic males of a polytypic poison-dart frog. Behav Ecol Sociobiol 68:249–261. https://doi.org/10.1007/s00265-013-1640-4
Winkler DW (1987) A general model for parental care. Am Nat 130(4):526–543. https://doi.org/10.1086/284729
Wong B, Candolin U (2015) Behavioral responses to changing environments. Behav Ecol 26(3):665–673. https://doi.org/10.1093/beheco/aru183
Wong BB, Candolin U, Lindström K (2007) Environmental deterioration compromises socially enforced signals of male quality in three-spined sticklebacks. Am Nat 170(2):184–189. https://doi.org/10.1086/519398
Yang Y, Blomenkamp S, Dugas MB, Richards-Zawacki CL, Pröhl H (2019) Mate choice versus mate preference: inferences about color-assortative mating differ between field and lab assays of poison frog behavior. Am Nat 193(4):598–607. https://doi.org/10.1086/702249
Acknowledgements
BR is grateful to Matthieu Paquet, Mylene Mariette, Susana Varela, and Rita Covas for the invitation to participate in this special issue. We would like to thank Ria Sonnleitner for her insightful comments on the manuscript, Mileidy Betancourth for sharing her thoughts on the potential impact of global change on poison frog territoriality, Andrius Pašukonis for making his amphibian vector drawings publicly available, Jennifer Devillechabrolle for her help getting photos illustrating gold mining activities, and two anonymous referees for their positive and thoughtful feedback. Many thanks to Mileidy Betancourth, Justin P. Lawrence, Bernard Gissinger, Alexandre David, and Fernando Vargas-Salinas for allowing us to use their photographs.
Funding
Open Access funding provided by University of Jyväskylä (JYU).
Author information
Authors and Affiliations
Contributions
BR conceived the paper; LS-J wrote the first draft; CF designed the conceptual overview figure. All the authors discussed the ideas, contributed to the following drafts, and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlippe Justicia, L., Fouilloux, C.A. & Rojas, B. Poison frog social behaviour under global change: potential impacts and future challenges. acta ethol 26, 151–166 (2023). https://doi.org/10.1007/s10211-022-00400-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-022-00400-6