Abstract
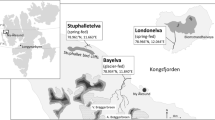
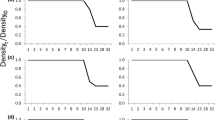
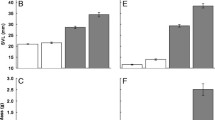
Plasticity in the larval life history of the Ezo salamander Hynobius retardatus has been reported. In the present study, we monitored larvae of this salamander species in a fragmented forest in the environs of Sapporo, Japan. Overwintering larvae were detected in one of the two ponds examined, i.e., in the pond that was permanent, with water supplied from several spring-fed points. Four seasonal transition phases in water temperature were observed because of the abundant spring-fed groundwater supply and canopy cover from May to October. These phases included a warming period, a constant-high period, a cooling period, and a constant-low period. During the constant-low period, premetamorphic larvae that had already emerged during the cooling period were continuously detected; however, the composition of the developmental stages remained unchanged, with larval growth progressing slowly. There is apparently a critical temperature that represents the threshold for metamorphosis initiation. The critical temperature is expected to be slightly higher than the groundwater temperature at the spring-fed points. Emigration of overwintered larvae resumed during the warming period and continued during the constant-high period in the year following hatching. In the nearby temporary pond, the one-year-old cohort completed metamorphosis during the summer of the hatch year.







Similar content being viewed by others
References
Alvarez D, Nicieza AG (2002) Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct Ecol 16:640–648
Arai T (2009) Climate change and variations in the water temperature and ice cover of inland waters. Jpn J Limnol 70:99–116
Arai T, Wakahara M (1993) Hemoglobin transition from larval to adult types in normally metamorphosing, metamorphosed and metamorphosis-arrested Hynobius retardatus. Zool Sci 10:637–644
Beachy CK (1995) Effects of larval growth history on metamorphosis in a stream-dwelling salamander (Desmognathus ochrophaeus). J Herpetol 29:375–382
Bizer JR (1978) Growth rates and size at metamorphosis of high elevation populations of Ambystoma tigrinum. Oecologia 34:175–184
Boone MD, Scott DE, Niewiarowski PH (2002) Effects of hatching time for larval ambystomatid salamanders. Copeia 2002:511–517
Bruce RC (1982) Larval periods and metamorphosis in two species of salamanders of the genus Eurycea. Copeia 117–127
Bruce RC (1985) Larval period and metamorphosis in the salamander Eurycea bislineata. Herpetologica 41:19–28
Collins JP (1979) Intrepopulation variation in the body size at metamorphosis and timing of metamorphosis in the bullfrog, Rana catesbeiana. Ecolgy 60:738–749
Frieden E, Wahlborg A, Howard E (1965) Temperature control of the response of tadpoles to triiodothyronine. Nature 205:1173–1176
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Holbrook JD, Dorn NJ (2016) Fish reduce anuran abundance and decrease herpetofaunal species richness in wetlands. Freshw Biol 61:100–109
Iwasaki F, Wakahara M (1999) Adaptable larval life histories in different populations of the salamander, Hynobius retardatus, living in various habitats. Zool Sci 16:667–674
Iwasawa H, Yamashita K (1991) Normal stages of development of a hynobiid salamander Hynobius nigrescens Stejneger. Jpn J Herpetol 14:39–62
Kishida O, Costa Z, Tezuka A, Michimae H (2014) Inducible offences affect predator–prey interactions and life-history plasticity in both predators and prey. J Anim Ecol 83:899–906
Krause ET, Steinfartz S, Caspers BA (2011) Poor nutritional conditions during the early larval stage reduce risk-taking activities of fire salamander larvae (Salamandra salamandra). Ethology 117:416–421
Kukita S, Gouda M, Ikeda S, Ishibashi S, Furuya T, Nakamura K (2015) Effects of photoperiod and temperature on growth and development in clouded salamander (Hynobius nebulosus) larvae. Zool Sci 32:266–271
Kusano T (1981) Growth and survival rate of the larvae of Hynobius nebulosus tokyoensis Tago (Amphibia, Hynobiidae). Res Popul Ecol 23:360–378
Laurila A, Kujasalo J (1999) Habitat duration, predation risk and phenotypic plasticity in common frog (Rana temporaria) tadpoles. J Anim Ecol 68:1123–1132
Merila J, Laurila A, Laugen AT, Rasanen K, Pahkala M (2000a) Plasticity in age and size at metamorphosis in Rana temporaria: comparison of high and low latitude populations. Ecography 23:457–465
Merila J, Laurila A, Pahkala M, Rasanen K, Laugen AT (2000b) Adaptive phenotypic plasticity in timing of metamorphosis in the common frog Rana temporaria. Ecoscience 7:18–24
Michimae H (2011) Plasticity in the timing of a major life-history transition and resulting changes in the age structure of populations of the salamander Hynobius retardatus. Biol J Linn Soc 102:100–114
Michimae H, Emura T (2012) Correlated evolution of phenotypic plasticity in metamorphic timing. J Evol Biol 25:1331–1339
Michimae H, Wakahara M (2002) A tadpole-induced polyphenism in the salamander Hynobius retardatus. Evolution 56:2029–2038
Misawa Y, Matsui M (1997) Larval life history variation in two populations of the Japanese salamander Hynobius kimurae (Amphibia, Urodela). Zool Sci 14:257–262
Mochizuki K, Goda T, Yamauchi K (2012) Gene expression profile in the liver of Rana catesbeiana tadpoles exposed to low temperature in the presence of thyroid hormone. Biochem Biophys Res Commun 420:845–850
Moriya T (1983a) The effect of temperature on the action of thyroid hormone and prolactin in larvae of the salamander Hynobius retardatus. Gen Comp Endocrinol 49:1–7
Moriya T (1983b) Cytological changes induced by low temperature in the thyroid glands of larvae of the salamander Hynobius retardatus. Gen Comp Endocrinol 49:8–14
Murata T, Yamauchi K (2005) Low-temperature arrest of the triiodothyronine-dependent transcription in Rana catesbeiana red blood cells. Endocrinology 146:256–264
Nakano S, Kitano F, Maekawa K (1996) Potential fragmentation and loss of thermal habitats for charrs in the Japanese archipelago due to climatic warming. Freshw Biol 36:711–722
Newman RA (1998) Ecological constraints on amphibian metamorphosis: interactions of temperature and larval density with responses to changing food level. Oecologia 115:9–16
Nishihara-Takahashi A (1999) Faster growth of head size of pre-feeding larvae in a cannibalistic population of the salamander Hynobius retardatus. Zool Sci 16:303–307
Nishikawa K, Matsui M (2008) A comparative study on the larval life history in two populations of Hynobius boulengeri from Kyushu, Japan (Amphibia: Urodela). Curr Herpetol 27:9–22
Ohdachi S (1994) Growth, metamorphosis, and gape-limited cannibalism and predation on tadpoles in larvae of salamanders Hynobius retardatus. Zool Sci 11:127–131
Orizaola G, Dahl E, Nicieza AG, Laurila A (2013) Larval life history and anti-predator strategies are affected by breeding phenology in an amphibian. Oecologia 171:873–881
Phillips CA, Johnson JR, Dreslik MJ, Petzing JE (2002) Effects of hydroperiod on recruitment of mole salamanders (genus Ambystoma) at a temporary pond in Vermilion County, Illinois. Trans Ill Acad Sci 95:131–139
Rose CS (2005) Integrating ecology and developmental biology to explain the timing of frog metamorphosis. Trends Ecol Evol 20:129–135
Rowe CL, Dunson WA (1995) Impacts of hydroperiod on growth and survival of larval amphibians in temporary ponds of central Pennsylvania, USA. Oecologia 102:397–403
Scott DE (1990) Effects of larval density in Ambystoma opacum an experiment in large scale field enclosures. Ecology 71:296–306
Segev O, Blaustein L (2007) Priority effects of the early breeding fire salamander on the late breeding banded newt. Hydrobiologia 583:275–283
Semlitsch RD, Scott DE, Pechmann JHK, Gibbons JW (1996) Structure and dynamics of an amphibian community: evidence from a 16-year study of a natural pond. In: Cody ML, Smallwood JA (eds) Long-term studies of vertebrate communities. Academic, San Diego, pp 217–248
Skelly DK, Freidenburg LK, Kiesecker JM (2002) Forest canopy and the performance of larval amphibians. Ecology 83:983–992
Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113:563–586
Taylor AC, Kollros JJ (1946) Stages in the normal development of Rana pipiens larvae. Anat Rec 94:7–23
Timm BC, McGarigal K, Gamble LR (2007) Emigration timing of juvenile pond-breeding amphibians in western Massachusetts. J Herpetol 41:243–250
Van Buskirk J, Yurewicz KL (1998) Effects of predators on prey growth rate: relative contributions of thinning and reduced activity. Oikos 82:20–28
Voss SR (1993) Relationship between stream order and length of larval period in the salamander Eurycea wilderae. Copeia 1993:736–742
Wilbur HM (1987) Regulation of structure in complex systems experimental temporary pond communities. Ecology 68:1437–1452
Wissinger SA, Whiteman HH, Denoel M, Mumford ML, Aubee CB (2010) Consumptive and nonconsumptive effects of cannibalism in fluctuating age-structured populations. Ecology 91:549–559
Acknowledgments
We would like to thank Dr. Masami Wakahara for helpful discussions. We thank the members of our laboratory for supporting the field survey.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Toshifumi Minamoto.
Rights and permissions
About this article
Cite this article
Midori, T., Kuwahara, T. & Yamashiki, N. Retardation of larval development in the salamander Hynobius retardatus in a permanent pond with abundant spring water. Limnology 18, 287–299 (2017). https://doi.org/10.1007/s10201-016-0506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-016-0506-7