Abstract
Background
Focal-onset seizures (FOS) are commonly experienced by people with epilepsy and have a significant impact on quality of life (QoL). This study aimed to develop a mapping algorithm to predict SF-6D values in adults with FOS for use in economic evaluations of a new treatment, cenobamate.
Methods
An online survey, including questions on disease history, SF-36, and an epilepsy-specific measure (QOLIE-31-P) was administered to people with FOS in the UK, France, Italy, Germany, and Spain. A range of regression models were fitted to SF-6D scores including direct and response mapping approaches.
Results
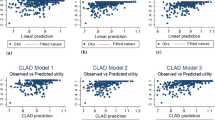
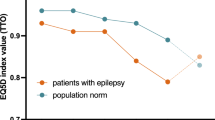
361 individuals were included in the analysis. In the previous 28 days, the mean number of FOS experienced was 3, (range 0–43) and the mean longest period of consecutive days without experiencing a seizure was 14 days (range 1–28 days or more). Mean responses on all SF-36 dimensions were lower than general population norms. Mean SF-6D and QOLIE-31-P scores were 0.584 and 45.72, respectively. The best performing model was the ordinary least squares (OLS), with root mean squared error and mean absolute error values of 0.0977 and 0.0742, respectively. Explanatory variables which best predicted SF-6D included seizure frequency, severity, freedom, and age.
Conclusion
People with uncontrolled FOS have poor QoL. The mapping algorithm enables the prediction of SF-6D values from clinical outcomes in people with FOS. It can be applied to outcome data from clinical trials to facilitate cost-utility analysis.


Similar content being viewed by others
Data availability
Not available.
Code availability
Not available.
References
World Health Organization. Epilepsy: a public health imperative: summary. World Health Organization, (2019). https://apps.who.int/iris/handle/10665/325440
Scharfman, H.T.: The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 7(4), 348–354 (2007). https://doi.org/10.1007/s11910-007-0053-z
Fisher, R.S.: The new classification of seizures by the international league against epilepsy. Curr. Neurol. Neurosci. Rep. 17(6), 48 (2017). https://doi.org/10.1007/s11910-017-0758-6
Birbeck, G.L., et al.: Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia 43(5), 535–538 (2002)
Choi, H., Hamberger, M.J., Munger, C.H., et al.: Seizure frequency and patient-centered outcome assessment in epilepsy. Epilepsia 55(8), 1205–1212 (2014). https://doi.org/10.1111/epi.12672
Luoni, C., et al.: Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia 52(12), 2181–2191 (2011). https://doi.org/10.1111/j.1528-1167.2011.03325.x
Mukuria, C., Young, T., Keetharuth, A., et al.: Sensitivity and responsiveness of the EQ-5D-3L in patients with uncontrolled focal seizures: an analysis of phase III trials of adjunctive brivaracetam. Qual. Life Res. 26(3), 749–759 (2017). https://doi.org/10.1007/s11136-016-1483-3
Velez, F.F., Bond, T.C., Anastassopoulos, K.P., et al.: Impact of seizure frequency reduction on health-related quality of life among clinical trial subjects with refractory partial-onset seizures: a pooled analysis of phase III clinical trials of eslicarbazepineacetate. Epilepsy Behav. 68, 203–207 (2017). https://doi.org/10.1016/j.yebeh.2016.10.027
Cramer, J.A., Perrine, K., Devinsky, O., et al.: Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia 39(1), 81–88 (1998). https://doi.org/10.1111/j.1528-1157.1998.tb01278.x
Viteva, E.L.: Seizure frequency and severity: How really important are they for the quality of life of patients with refractory epilepsy. Ann. Indian Acad. Neurol. 17(1), 35–42 (2014). https://doi.org/10.4103/0972-2327.128544
Guyatt, G.H., Feeny, D.H., Patrick, D.L.: Measuring health-related quality of life. Ann. Intern. Med. 118(8), 622–629 (1993). https://doi.org/10.7326/0003-4819-118-8-199304150-00009
Longworth, L., Yang, Y., Young, T., Mulhern, B., Hernandez, A.M., Mukuria, C., Rowen, D., Tosh, J., Tsuchiya, A., Evans, P., Davianee, K.A., Brazier, J.: Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol. Access 18(9), 1–224 (2014). https://doi.org/10.3310/hta18090
Longworth, L., Rowen, D.: Mapping to obtain EQ-5D utility values for use in NICE health technology assessments. Value Health 16(1), 202–210 (2013). https://doi.org/10.1016/j.jval.2012.10.010
Maresh, M.J., Holmes, V.A., Patterson, C.C., Young, I.S., Pearson, D.W.M., Walker, J.D., McCance, D.R.: Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care 38(1), 34–42 (2015). https://doi.org/10.2337/dc14-1755
Krauss, G.L., Klein, P., Brandt, C., Lee, S.K., Milanov, I., Milovanovic, M., Kamin, M., Steinhoff, B.J.: Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 19(1), 38–48 (2020). https://doi.org/10.1016/s1474-4422(19)30399-0
Chung, S.S., French, J.A., Kowalski, J., Krauss, G.L., Lee, S.K., Maciejowski, M., Rosenfeld, W.E., Sperling, M.R., Mizne, S., Kamin, M.: Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology 94(22), e2311–e2322 (2020). https://doi.org/10.1212/WNL.0000000000009530
Brazier, J., Roberts, J., Deverill, M.: The estimation of a preference-based measure of health from the SF-36. J. Health Econ. 21(2), 271–292 (2002). https://doi.org/10.1016/s0167-6296(01)00130-8
Optum PROCoRE 1.5 Smart Measurement® System December 2019. Optum, Inc 1301 Atwood Avenue, Suite 311 N Johnston, R.I. 02919, U.S.A (2018)
Brazier, J.E., Roberts, J.: The estimation of a preference-based measure of health from the SF-12. Med. Care (2004). https://doi.org/10.1097/01.mlr.0000135827.18610.0d
Brazier, J.E., Hanmer, J., Rowen, D.: Revised SF-6D scoring programmes: a summary of improvements. PRO Newslett. 40, 14–15 (2008)
Guide to the methods of technology appraisal. National Institute for Health and Clinical Excellence (NICE) London, UK. (2013). http://www.nice.org.uk/process/pmg9. Accessed 16th Mar 2021
Sanghera, S., Coast, J.: Measuring quality-adjusted life-years when health fluctuates. Value Health 23(3), 343–350 (2020). https://doi.org/10.1016/j.jval.2019.09.2753
Wijnen, B.F.M., Mosweu, I., Majoie, M.H.J.M., Ridsdale, L., de Kinderen, R.J.A., Evers, S.M.A.A., McCrone, P.: A comparison of the responsiveness of EQ-5D-5L and the QOLIE-31P and mapping of QOLIE-31P to EQ-5D-5L in epilepsy. Eur. J. Health Econ. 19(6), 861–870 (2018). https://doi.org/10.1007/s10198-017-0928-0
Longworth, L., et al.: Estimating utility data from clinical indicators for patients with stable angina. Eur. J. Health Econ. 6(4), 347–353 (2005)
Poole, C.D., et al.: A comparison of physician-rated disease severity and patient reported outcomes in mild to moderately active ulcerative colitis. J. Crohns Colitis 4(3), 275–282 (2010)
Abdin, E., et al.: Mapping the positive and negative syndrome scale scores to EQ-5D-5L and SF-6D utility scores in patients with schizophrenia. Qual. Life Res. 28(1), 177–186 (2019)
Ghatnekar, O., Eriksson, M., Glader, E.-L.: Mapping health outcome measures from a stroke registry to EQ-5D weights. Health Qual. Life Outcomes 11(1), 34 (2013)
Chavez, L.J., et al.: Preference weights for the spectrum of alcohol use in the U.S. population. Drug Alcohol. Depend. 161, 206–213 (2016)
Cramer, J.A., Rajagopalan, K., Anastassopoulos, K.P., Blum, D., et al.: Health-related quality of life in patients treated with eslicarbazepine acetate monotherapy: pooled analysis from two registered clinical trials. Epilepsy Behav. 92, 31–35 (2019). https://doi.org/10.1016/j.yebeh.2018.12.003
Deleo, F., et al.: The impact of perampanel treatment on quality of life and psychiatric symptoms in patients with drug-resistant focal epilepsy: an observational study in Italy. Epilepsy Behav. 99, 106391 (2019). https://doi.org/10.1016/j.yebeh.2019.06.034
Nakhutina, L., Kunnakkat, S.D., Coleman, M., Lushbough, C., Arnedo, V., Soni, N., Grant, A.C.: Effects of adjunctive lacosamide on mood and quality of life in patients with epilepsy. Epilepsy Behav. 73, 90–94 (2017). https://doi.org/10.1016/j.yebeh.2017.05.001
Sancho, J., et al.: Changes in seizure severity and quality of life in patients with refractory partial epilepsy. Epilepsy Behav. 19(3), 409–413 (2010). https://doi.org/10.1016/j.yebeh.2010.08.011
Gray, L.A., Alava, M.H.: A command for fitting mixture regression models for bounded dependent variables using the beta distribution. Stand. Genomic Sci. 18(1), 51–75 (2018)
Hernández-Alava, M., Wailoo, A.: ALDVMM: a command for fitting adjusted limited dependent variable mixture models to EQ-5D. Stand. Genomic Sci. 15(3), 737–750 (2015). https://doi.org/10.1177/1536867X1501500307
Abellán Perpiñán, J.M., Sánchez Martínez, F.I., Martínez Pérez, J.E., Méndez, I.: Lowering the “floor” of the Sf-6d scoring algorithm using a lottery equivalent method. Health Econ. 21(11), 1271–1285 (2012)
Maruish, M.E. (ed.) User’s manual for the SF-36v2 Health Survey (3rd edn). Lincoln, RI: QualityMetric Incorporated
Browne, C., Brazier, J., Carlton, J., Alavi, Y., Jofre-Bonet, M.: Estimating quality-adjusted life years from patient-reported visual functioning. Eye (Lond) 26(10), 1295–1301 (2012). https://doi.org/10.1038/eye.2012.137
Collado-Mateo, D., Chen, G., Garcia-Gordillo, M.A., et al.: Fibromyalgia and quality of life: mapping the revised fibromyalgia impact questionnaire to the preference-based instruments. Health Qual Life Outcomes 15(1), 114 (2017). https://doi.org/10.1186/s12955-017-0690-0
Siani, C., de Peretti, C., Millier, A., Boyer, L., Toumi, M.: Predictive models to estimate utility from clinical questionnaires in schizophrenia: findings from EuroSC. Qual Life Res 25, 925–934 (2016). https://doi.org/10.1007/s11136-015-1120-6
Langfitt, J.T., et al.: Validity and responsiveness of generic preference-based HRQOL instruments in chronic epilepsy. Qual Life Res 15(5), 899–914 (2006)
Funding
PHMR received financial support from Arvelle Therapeutics GmbH for conducting this study, including the development, administration and data collection of the online survey, development of the mapping algorithms and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
IF: Led the analysis and drafting of the manuscript, contributed to the study design and interpretation. JM: Contributed to the study design, analysis, interpretation and drafting of the manuscript. EDO’F: Contributed to the study design, analysis, interpretation and drafting of the manuscript. EAB: Contributed to the study design, analysis, interpretation and drafting of the manuscript. KT: Contributed to the study design, analysis, interpretation and drafting of the manuscript. NS: Contributed to the study design, analysis, interpretation and drafting of the manuscript. AM: Contributed to the analysis, interpretation and drafting of the manuscript. LL: Led the study design, contributed to the analysis, interpretation and drafting of the manuscript. Oversight of the project.
Corresponding author
Ethics declarations
Conflict of interest
I. Flint, A. Meunier, and Dr L. Longworth are employees at PHMR. PHMR received financial support from Arvelle Therapeutics GmbH for the work, including the development, administration and data collection of the online survey, development of the mapping algorithms and preparation of the manuscript. J. Medjedovic; E. Drogon O’Flaherty; N. Savic and E. Alvarez-Baron are full-time employees of Arvelle Therapeutics GmbH who funded the work. K. Thangavelu of MeDaStats LLC is a consultant statistician to Arvelle Therapeutics GmbH.
Ethical approval
The study protocol and survey materials were approved by an independent ethical reviewer prior to the start of the interviews. The research ethics expert was working under the auspices of the Association of Research Managers and Administrators (https://arma.ac.uk/).
Consent to participate
Before the respondent started the online survey, an information sheet was provided which included a breakdown of each study component and explained why the individual’s input was important for the study’s success; how the data would be managed; and their data protection rights. Following this, participants were issued an electronic informed consent form which they had to read and agree to before proceeding to the survey. Individuals who did not provide consent at this stage were unable to proceed to the survey.
Consent for publication
Before the respondent started the survey, an information sheet was provided which informed them of the potential use of anonymised data for publication in medical journals, conference presentations or which would be provided to health care decision makers. All participants provided informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Flint, I., Medjedovic, J., Drogon O’Flaherty, E. et al. Mapping analysis to predict SF-6D utilities from health outcomes in people with focal epilepsy. Eur J Health Econ 24, 1061–1072 (2023). https://doi.org/10.1007/s10198-022-01519-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-022-01519-w