Abstract
Objective
The goal of this study was to assess the differences between an ex ante and an ex post cost-effectiveness analysis of Dabigatran etexilate vs VKAs for the prevention of thromboembolic events in non-valvular atrial fibrillation patients and to draw lessons on the design and use of real-world data for decision making.
Methods
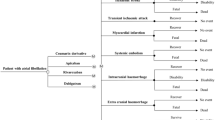
The same model was used to calculate the cost-effectiveness ratio using two sets of parameters. One set included the efficacy and safety outcomes data from RE-LY, the pivotal trial comparing Dabigatran to warfarin; cost data came from an ex ante publication. Outcomes data for the second set came from real-world data studies. Cost data were a mix of real-world data and other sources. Two treatment strategies were compared: treatment initiation by either Dabigatran or VKAs, followed by either VKAs or Dabigatran. A crude comparison of results was performed; the impact of data differences was then assessed. Probabilistic sensitivity results of the two analyses were compared.
Results
With real-world evidence, Dabigatran at both dosages was more effective for the prevention of ischemic strokes, intra-cranial haemorrhages, with less major extra-cranial haemorrhages and a similar risk of myocardial infarction. Using clinical trial data, Dabigatran150 mg (resp. Dabigatran110 mg) as a first-line treatment vs VKAs yielded an ICER of € 8077/QALY (resp. € 13,116/QALY). Real-world evidence scenarios were cost-saving and more effective for both dosages.
Conclusion
The reassessment of outcomes and cost data had an impact on results, improving the efficiency of Dabigatran. We identify methodological issues which should be discussed if post-launch RWE based cost-effectiveness data become a standard in HTA decision making.





Similar content being viewed by others
References
Garrison, L.P., Neumann, P.J., Erickson, P., et al.: Using real-world data for coverage and payment decisions: the ISPOR real-world data task force report. Value Health 10, 326–335 (2006)
Connolly, S.J., Ezekowitz, M.D., Yusuf, S., Eikelboom, J., Oldgren, J., Parekh, A., Pogue, J., Reilly, P.A., Themeles, E., Varrone, J., Wang, S., Alings, M., Xavier, D., Zhu, J., Diaz, R., Lewis, B.S., Darius, H., Diener, H.C., Joyner, C.D., Wallentin, L.: RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361(12), 1139–1151 (2009). https://doi.org/10.1056/nejmoa0905561
Chevalier, J., Delaitre, O., Hammès, F., de Pouvourville, G.: Cost-effectiveness of Dabigatranversus vitamin K antagonists for the prevention ofstroke in patients with atrial fibrillation: a French payer perspective. Arch. Cardiovasc. Dis. 107(6–7), 381–390 (2014)
Lanitis, T., Cotté, F.E., Gaudin, A.F., Kachaner, I., Kongnakorn, T., Durand-Zaleski, I.: Stroke prevention in patients with atrial fibrillation in France: comparative cost-effectiveness of new oral anticoagulants (Apixaban, Dabigatran, and Rivaroxaban), warfarin, and aspirin. J. Med. Econ. 17(8), 587–598 (2014)
Sorensen, S.V., Kansal, A.R., Connolly, S., Peng, S., Linnehan, J., Bradley-Kennedy, C., Plumb, J.M.: Cost-effectiveness of Dabigatranetexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb. Haemost. 105, 908–919 (2011)
Kansal, A.R., Sharma, M., Bradley-Kennedy, C., et al.: Dabigatran versus Rivaroxaban for the prevention of stroke and systemic embolism in atrial fibrillation in Canada. Comparative efficacy and cost-effectiveness. Thromb. Haemost. 108(4), 672–682 (2012)
Quinn, T.J., Dawson, J., Walters, M.R., Lees, K.R.: Functional outcome measures in contemporary stroke trials. Int. J. Stroke 4(3), 200–205 (2009)
Fang, M.C., Go, A.S., Hylek, E.M., et al.: Age and the risk of warfarin-associated hemorrhages: the anticoagulation and risk factors in atrial fibrillation study. J. Am. Geriatr. Soc. 54, 1231–1236 (2006)
Blin, P., de Pouvourville, G., Cottin, Y., Dureau-Pournin, C., Abouelfath, A., Lassalle, R., Bénichou, J., Droz-Perroteau, C., Mismetti, P., Moore, N.: Comparative effectiveness and medical cost of Dabigatranversus vitamin K antagonists from ENGEL 2: a french nationwide cohort of 100.000 patients with non-valvular atrial fibrillation. Value Health 20, 9 (2017)
Blin, P., Dureau-Pournin, C., Cottin, Y., Bénichou, J., Mismetti, P., Abouelfath, A., Lassalle, R., Droz, C., Moore, N.: Effectiveness and safety of 110 or 150 mg Dabigatran versus vitamin K antagonists in non-valvular atrial fibrillation. Br. J. Clin. Pharmacol. (2018). https://doi.org/10.1111/bcp.13815
Blin, P., Dureau-Pournin, C., Bénichou, J., Cottin, Y., Mismetti, P., Abouelfath, A., Lassalle, R., Droz, C., Moore, N.: Comparative Real-Life Effectiveness and Safety of Dabigatranor Rivaroxaban vs. Vitamin K Antagonists: a High-Dimensional Propensity Score Matched New Users Cohort Study in the French National Healthcare Data System SNDS. Am. J. Cardiovasc. Drugs (2019). https://doi.org/10.1007/s40256-019-00359-z
Grimaldi-Bensouda, L., Le Heuzey, J.Y., Ferrières, J., Leys, D., Davy, J.M., Martinez, M., Smadja, D., Ellie, E., Sablot, D., Nighoghossian, N., Benichou, J., Touzé, E., Abenhaim, L.: Non-valvular atrial fibrillation, VKA and non-VKA anticoagulants and stroke: the Stroke Prevention and Anticoagulants (SPA) case-control study. Eur. Heart J. 38, 769–770 (2017)
Haute Autorité de Santé (HAS). Choix méthodologiques pour l’évaluation économique à la HAS. Guide méthodologique. http://www.has-sante.fr/portail/jcms/c_1120708/guide-choix-methodologiques-pour-l-evaluation-economique-a-la-has (2011). Accessed 31 Oct 2018
de Pouvourville G, Karam P. Le coût hospitalier des événements cérébro-vasculaires associés à une fibrillation atriale en France. J Gestion Eco Santé 5 (2019) (in press)
ATIH. ENC MCO. https://www.scansante.fr/referentiel-de-couts-mco-2015. Accessed 31 Oct 2018
ATIH. ENC MCO. https://www.scansante.fr/referentiel-de-couts-ssr-2015. Accessed 31 Oct 2018
Blin, P., Philippe, F., Bouée, S., Laurendeau, C., Torreton, E., Gourmelin, J., Leproust, S., Levy-Bachelot, L., Steg, P.G.: Outcomes following acute hospitalised myocardial infarction in France: an insurance claims database analysis. Int. J. Cardiol. 15(219), 387–393 (2016)
Ministère des Solidarités et de la Santé. Les dépenses de santé en 2017. Résultats des comptes de la santé-Edition. https://drees.solidarites-sante.gouv.fr/etudes-et-statistiques/publications/panoramas-de-la-drees/article/les-depenses-de-sante-en-2017-resultats-des-comptes-de-la-sante-edition-2018 (2018). Accessed 31 Oct 2018
Assurance Maladie. Coût des ALD en 2009. https://www.ameli.fr/l-assurance-maladie/statistiques-et-publications/donnees-statistiques/affection-de-longue-duree-ald/archives/cout-des-ald-en-2009.php (2009). Accessed 31 Oct 2018
ATIH: Les coûts en établissement d’hébergement pour personnes âgées dépendantes. https://www.atih.sante.fr/resultats-de-l-enquete-de-couts-ehpad-2013 (2013). Accessed 31 Oct 2018
Béjot, Y., Bailly, H., Graber, M., Garnier, L., Laville, A., Dubourget, L., Mielle, N., Chevalier, C., Durier, J., Giroud, M.: Impact of the ageing population on the burden of stroke: the Dijon Stroke Registry. Neuroepidemiology 52, 78–85 (2019)
Lecoffre, C., de Peretti, C., Gabet, A., Grimaud, O., Woimant, F., Giroud, M., et al.: L’accident vasculaire cérébral en France: patients hospitalisés pour AVC en 2014 et évolutions 2008–2014. BEH 5, 84–94 (2017)
Pouvourville, G.: Anti-coagulants d’action directe: une revue de la littérature des études coût-efficacité en Europe. Arch. Cardiovasc. Dis. 8, 180–191 (2016)
Harenberg, J., Marx, S., Obermann, K., Frölich, L., Wehling, M.: Comparison of cost-effectiveness of anticoagulation with Dabigatran, Rivaroxaban and Apixaban in patients with non-valvular atrial fibrillation across countries. J. Thromb. Thrombolysis 37(4), 507–523 (2014)
Kongnakorn, T., Lanitis, T., Lieven, A., Thijs, V., Marbaix, S.: Cost effectiveness of Apixaban versus aspirin for stroke prevention in patients with non-valvular atrial fibrillation in Belgium. Clin. Drug Investig. 34(10), 709–721 (2014)
Langkilde, L.K., Asmussen, B.M., Overgaard, M.: Cost-effectiveness of Dabigatranetexilate for stroke prevention in non-valvular atrial fibrillation. Applying RE-LY to clinical practice in Denmark. J. Med. Econ. 15(4), 695–703 (2012)
González-Juanatey, J.R., Álvarez-Sabin, J., Lobos, J.M., Martínez-Rubio, A., Reverter, J.C., Oyagüez, I., González-Rojas, N., Becerra, V.: Cost-effectiveness of Dabigatranfor stroke prevention in non-valvular atrial fibrillation in Spain. Rev. Esp. Cardiol. 65(10), 901–910 (2012)
Rognoni, C., Marchetti, M., Quaglini, S., Liberato, N.L.: Apixaban, Dabigatran, and Rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin. Drug Investig. 34(1), 9–17 (2014)
Verhoef, T., Redekop, W.K., Hasrat, F., de Boer, A., Maitland-van der Zee, A.H.: Cost effectiveness of new oral anticoagulants for stroke prevention in patients with atrial fibrillation in two different European healthcare settings. Am. J. Cardiovasc. Drugs 14(6), 451–462 (2014)
Silva Miguel, L., Rocha, E., Ferreira, J.: Economic evaluation of Dabigatranfor stroke prevention in patients with non-valvular atrial fibrillation. Rev. Port. Cardiol. 32(7–8), 557–565 (2013)
Kansal, A.R., Sorensen, S.V., Gani, R., Robinson, P., Pan, F., Plumb, J.M., Cowie, M.R.: Cost-effectiveness of dabigatranetexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 98(7), 573–578 (2012)
Pink, J., Lane, S., Pirmohamed, M., Hughes, D.A.: Dabigatranetexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ (2011). https://doi.org/10.1136/bmj.d6333
Davidson, T., Husberg, M., Janzon, M., Oldgren, J., Levin, L.Å.: Cost-effectiveness of dabigatran compared with warfarin for patients with atrial fibrillation in Sweden. Eur. Heart J. 34(3), 177–183 (2013)
Pletscher, M., Plessow, R., Eichler, K., Wieser, S.: Cost-effectiveness of dabigatranfor stroke prevention in atrial fibrillation in Switzerland. Swiss Med. Wkly. 8, 143 (2013). https://doi.org/10.4414/smw.2013.13732
Acknowledgements
Our thanks to Professor Maurice Giroud and Professor Yannick Béjot, Dijon Stroke Registry, for a free access to data; Professor Ariel Cohen, Hôpital Saint-Antoine, for his informed advice on clinical assumptions in the model; Katell Le Lay, Laura Luciani, Clément Le Diser, Samuel Seksik, from Boehringer Ingerlheim, for contributions and comments on the analysis and manuscript; Caroline Guilmet, François Bourhis, from Mapi Values, for their contributions in the adaptation of the model. Bruno Detournay, CEMKA, and Stephane Roze, HEVA HEOR for relevant comments on the first versions of the manuscript. The study was funded by a Boehringer Ingelheim grant to ESSEC Business School.
Funding
This research was funded by Boehringer Ingelheim France (FR) Grant number [316097 ADA -07/24/2017].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Pouvourville, G., Blin, P. & Karam, P. The contribution of real-world evidence to cost-effectiveness analysis: case study of Dabigatran etexilate in France. Eur J Health Econ 21, 235–249 (2020). https://doi.org/10.1007/s10198-019-01123-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-019-01123-5