Abstract
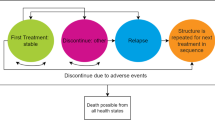
Second-generation atypical antipsychotics such as clozapine, olanzapine, risperidone, quetiapine, ziprasidone, amisulpride and ariprazole offer the potential to reduce the significant health care resource demands in the treatment of schizophrenia through improved levels of initial clinical response and reduced levels of long-term acute relapse. However, the optimal sequencing of these drugs remains unclear. To consider this issue from a health economic viewpoint a decision model approach was used comparing healthcare costs and clinical outcomes when treating patients with alternative sequences of atypical antipsychotic treatment. Treated patients were assumed to be in a current acute episode with at least a 10-year history of disease and to be naive to previous atypical treatments. Treatment strategies were based on either first-line olanzapine or risperidone with switching to the alternative drug as second-line treatment following an inadequate clinical response to first-line drug therapy. Clinical response data were derived from a pivotal published comparative study of both olanzapine and risperidone. Published data on the long-term use of antipsychotic drugs where used wherever possible to populate the model for relapse rates during the maintenance phase. Health care resource data were defined for Germany based on expert clinical opinion. A treatment strategy of first-line olanzapine was shown to be cost saving over a 1-year period, with additional clinical benefits in the form of avoided relapses. The model suggests that over the first year of treatment a strategy of first-line olanzapine is associated with lower risk of additional relapse (0.33 fewer acute relapses per 100 patients per year) and with cost savings (€35,306 per 100 patients per year). There is a need for longer term direct in-trial comparisons of atypical antipsychotics to confirm these indicative results.


Similar content being viewed by others
References
Goldner EM, Hsu L, Waraich P, Somers JM (2002) Prevalence and incidence studies of schizophrenic disorders: a systematic review of the literature. Can J Psychiatry 47:833–843
Knapp M (1997) Costs of schizophrenia. Br J Psychiatry 171:509–518
Rice DP (1999) The economic impact of schizophrenia. J Clin Psychiatry 60 [Suppl 1]:4–6
Garattini L, Rossi C, Tediosi F, Cornaggia C, Covelli G, Barbui C, Parazzini F (2001) Direct costs of schizophrenia in Italian community psychiatric services. Pharmacoeconomics 19:1217–1225
Salize HJ (2001) Costs of schizophrenia—what we know (not)? Psychiatr Prax 28 [Suppl 1]:21–28
Schulenburg JM Graf von der, Uber A, Höffler J, Trenckmann U, Kissling W, Seemann U, Müller P, Rüther E (1998) Untersuchungen zu den direkten und indirekten Kosten der Schizophrenie: eine empirische Analyse. Gesundh Okon Qual Manage 381–87
Gerlach J (1999) The continuing problem of extrapyramidal symptoms: strategies for avoidance and effective treatment. J Clin Psychiatry 60 [Suppl 23]:20–24
National Institute of Clinical Excellence NICE (2002) Guidance on the use of newer (atypical) antipsychotic drugs for the treatment of schizophrenia. Technology appraisal guidance no 43, sect 1.2. NICE: London
Tandon R (2002) Safety and tolerability: how do newer generation “atypical” antipsychotics compare? Psychiatr Q 73:297–311
Remington G, Kapur S (2000) Atypical antipsychotics: are some more atypical than others? Psychopharmacology (Berl) 148:3–15
Palmer CS, Revicki DA, Genduso LA, Hamilton SH, Brown RE (1998) A cost-effectiveness clinical decision analysis model for schizophrenia. Am J Manage Care 4:345–355
Almond S, O’Donnell O (2000) Cost analysis of the treatment of schizophrenia in the UK. A simulation model comparing olanzapine, risperidone and haloperidol. Pharmacoeconomics 17:383–389
Alexeyeva I, Mauskopf J, Earnshaw S, Stauffer VL, Gibson JP, Ascher-Svanum H, Ramsey J (2001) Comparing olanzapine and ziprasidone in the treatment of schizophrenia: a case study in modelling. J Med Econ 4:179–192
Tran PV, Dellva MA, Tollefson GD, Wentley AL, Beasley CM Jr (1998) Oral olanzapine versus oral haloperidol in the maintenance treatment of schizophrenia and related psychoses. Br J Psychiatry 172:499–505
Tran PV, Hamilton SH, Kuntz AJ, Potvin JH, Andersen SW, Beasley C Jr, Tollefson GD (1997) Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol 17:407–418
Tollefson GD, Beasley CM Jr, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME (1997) Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 154:466–474
Gureje O, Miles W, Keks N, Grainger D, Lambert T, McGrath J, Tran P, Catts S, Fraser A, Hustig H, Andersen S, Crawford AM (2003) Olanzapine vs risperidone in the management of schizophrenia: a randomized double-blind trial in Australia and New Zealand. Schizophr Res 61–:303–314
Meltzer HY, Okayli G (1995) Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry 152:183–190
Montes JM, Ciudad A, Gascon J, Gomez JC EFESO Study Group (2003) Safety, effectiveness, and quality of life of olanzapine in first-episode schizophrenia: a naturalistic study. Prog Neuropsychopharmacol Biol Psychiatry 27:667–674
Anonymous (2002) Rote Liste 2002 (Online). http://www.rote-liste.de, accessed November
Palmer CS, Revicki DA, Halpern MT, Hatziandreu EJ (1995) The cost of suicide and suicide attempts in the United States. Clinical Neuropharmacology 18 [Suppl 3]: S25–S33
Revicki DA, Shakespeare A, Kind P (1996) Preferences for schizophrenia-related health states: a comparison of patients, caregivers and psychiatrists. Int Clin Psychopharmacol 11:101–108
Glennie, Judith L (1997) Pharmacoeconomic evaluations of clozapine in treatment-resistant schizophrenia and risperidone in chronic schizophrenia—summary. Canadian Coordinating Office for Health Technology Assessment: Ottawa
Deckert C, Höffler J, Kortmann J, Linden M, Roth GD, Struck M, Clouth J, Czekalla J (2001) Cost-analysis of schizophrenia treatment in Germany. a comparison of olanzapine, risperidone and haloperidol using a clinical decision model. Gesundh Okon Qual Manage 6:161–166
Conflict of interest
No information supplied.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beard, S.M., Maciver, F., Clouth, J. et al. A decision model to compare health care costs of olanzapine and risperidone treatment for schizophrenia in Germany. Eur J Health Econ 7, 165–172 (2006). https://doi.org/10.1007/s10198-006-0347-0
Issue Date:
DOI: https://doi.org/10.1007/s10198-006-0347-0