Abstract
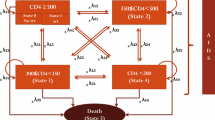
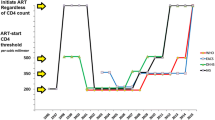
We evaluated the costs and effectiveness of starting highly active antiretroviral therapy (HAART) at different points during the course of HIV infection, defined on the basis of CD4 T-lymphocytes counts. The study considered 3,250 HAART-naive patients of the Italian Cohort Naive Antiretrovirals (ICONA), enrolled and followed between 1997 and 2002. In correspondence to the thresholds of 500, 350, and 200 CD4 cells/mm3, we selected immediate and deferred groups accounting for lead-time bias. The effects of immediate vs. deferred treatment on AIDS-free survival and direct health costs were estimated stratifying on the propensity score of immediate HAART initiation. The incremental cost-effectiveness ratio (ICER) and the cost-effectiveness acceptability curve were also obtained. Although immediate HAART initiation did not affect incidence AIDS and death at high CD4 levels, starting HAART with 200–349 CD4 cells/mm3 rather than deferring it below 200 CD4 cells/mm3, proved to be cost-effective.

Similar content being viewed by others
References
Shi M, Taylor JM, Currier RJ et al. (1996) Replacing time since human immunodeficiency virus infection by marker values in predicting residual time to acquired immunodeficiency syndrome diagnosis. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr Hum Retrovirol 12:309–316
Murphy EL, Collier AC, Kalish LA et al. (2001) Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med 135:17–26
Kaplan JE, Hanson D, Dworkin MS et al. (2000) Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 30 [Suppl]:S5–S14
Phillips AN, Cozzi-Lepri A, Lampe F et al. (2003) When should antiretroviral therapy be started for HIV infection? Interpreting the evidence from observational studies. AIDS 17:1863–1869
Egger M, May M, Chene G et al. (2002) Prognosis of HIV-1 infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 360:119–129
Opravil M, Ledergerber B, Hansjakob F et al. (2002) Clinical efficacy of early initiation of HAART in patients with asymptomatic HIV infection and CD4 cell count >350×106/l. AIDS 16:1371–1381
Kaplan JE, Hanson DL, Cohn DL et al. (2003) When to begin highly active antiretroviral therapy? Evidence supporting initiation of therapy at CD4+ lymphocyte counts <350 cells/microL. Clin Infect Dis 37:951–958
Monforte A, Arici C, Ippolito G et al. (1997) Lo studio di coorte I.CO.N.A. (Italian Cohort Naive Antiretrovirals): caratteristiche all’arruolamento dei primi 1676 soggetti. G Ital AIDS 8:119–126
U.S. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV infected adults and adolescents. Archived guidelines 1998–2002. Available at: http://www.aidsinfo.nih.gov/guidelines/archive.asp
Ahdieh-Grant L, Yamashita TE, Phair JP et al. (2003) When to initiate highly active antiretroviral therapy: a cohort approach. Am J Epidemiol 157:738–746
Cole S, Li R, Anastos K et al. (2004) Accounting for lead-time in cohort studies: evaluating when to initiate HIV therapies. Stat Med 23:3351–3363
Taylor JM, Law N (1998) Does the covariance structure matter in longitudinal modelling for the prediction of future CD4 counts? Stat Med 17:2381–2394
Marschner IC, Betensky RA, De Gruttola V et al. (1999) Clinical trials using HIV-1 RNA-based primary endpoints: statistical analysis and potential biases. J Acquir Immune Defic Syndr Hum Retrovirol 20:220–227
Polsky D, Glick HA, Willke R et al. (1997) Confidence intervals for cost-effectiveness ratios: a comparison of four methods. Health Econ 6:243–252
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman & Hall: New York:
Stinnet AA, Mullahy J (1998) A new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 18 [Suppl]:S68–S80
Hout BA van, Maiwenn JAL, Gordon GS et al. (1994) Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 3:309–319
O’Hagan A, Stevens JW, Montmartin J (2000) Inference for the cost-effectiveness acceptability curve and cost-effectiveness ratio. Pharmacoeconomics 17:339–349
Sabin CA, Phillips AN, Lee CA et al. (1994) Beta-2 microbulin as a predictor of prognosis in HIV-infected men with haemophilia: a proposed strategy for use in clinical care. Br J Haematol 86:366–371
Concorde Coordinating Committee (1994) Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Lancet 343:871–881
Sabin CA, Phillips AN (2001) Treatment comparisons in HIV infection: the benefits and limitations of observational cohort studies. J Antimicrob Chemother 47:371–375
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55
Lacey L, Mauskopf J, Lindrooth R et al. (1999) A prospective cost-consequence analysis of adding lamivudine to zidovudine-containing antiretroviral treatment regimens for HIV infection in the US. Pharmacoeconomics 15 S1:23–37
Acknowledgements
The ICONA network is supported by an unrestricted educational grant provided by GlaxoSmithKline Italy. The members of the ICONA Study Group are: Ancona: M. Montroni, G. Scalise, M.C. Braschi, M.S. Del Prete; Aviano: U. Tirelli, R. Cinelli; Bari: G. Pastore, N. Ladisa, G. Minafra; Bergamo: F. Suter, C. Arici; Bologna: F. Chiodo, V. Colangeli, C. Fiorini, O. Coronado; Brescia: G. Carosi, G.P. Cadeo, C. Torti, C. Minardi, D. Bertelli; Busto Arsizio: G. Rizzardini, G. Migliorino; Cagliari: P.E. Manconi, P. Piano; Catanzaro: T. Ferraro, A. Scerbo; Chieti: E. Pizzigallo, M. D’Alessandro; Como: D. Santoro, L. Pusterla; Cremona: G. Carnevale, D. Galloni; Cuggiono: P. Viganò, M. Mena; Ferrara: F. Ghinelli, L. Sighinolfi; Firenze: F. Leoncini, F. Mazzotta, M. Pozzi, S. Lo Caputo; Foggia: G. Angarano, B. Grisorio, A. Saracino, S. Ferrara; Galatina: P. Grima, P. Tundo; Genova: G. Pagano, G. Cassola, A. Alessandrini, R. Piscopo; Grosseto: M. Toti, S. Chigiotti; Latina: F. Soscia, L. Tacconi; Lecco: A. Orani, P. Perini; Lucca: A. Scasso, A. Vincenti; Macerata: F. Chiodera, P. Castelli; Mantova: A. Scalzini, G. Fibbia; Milan: M. Moroni, A. Lazzarin, A. Cargnel, G.M. Vigevani, L. Caggese, A. d’Arminio Monforte, D. Repetto, R. Novati, A. Galli, S. Merli, C. Pastecchia, M.C. Moioli; Modena: R. Esposito, C. Mussini; Napoli: N. Abrescia, A. Chirianni, C.M. Izzo, M. Piazza, M. De Marco, R. Viglietti, E. Manzillo, M. Graf; Palermo: A. Colomba, V. Abbadessa, T. Prestileo, S. Mancuso; Parma: C. Ferrari, P. Pizzaferri; Pavia: G. Filice, L. Minoli, R. Bruno, S. Novati; Perugia: F. Baldelli, M. Tinca; Pesaro: E. Petrelli, A. Cioppi; Piacenza: F. Alberici, A. Ruggieri; Pisa: F. Menichetti, C. Martinelli; Potenza: C. De Stefano, A. La Gala; Ravenna: G. Ballardini, E. Briganti; Reggio Emilia: G. Magnani, M.A. Ursitti; Rimini: M. Arlotti, P. Ortolani; Roma: R. Cauda, F. Dianzani, G. Ippolito, A. Antinori, G. Antonucci, S. D’Elia, P. Narciso, N. Petrosillo, V. Vullo, A. De Luca, S. Di Giambenedetti, M. Zaccarelli, R. Acinapura, P. De Longis, M. Ciardi, G. D’Offizi, MP Trotta, P. Noto, M. Lichtner, M.R. Capobianchi, E. Girardi, P. Pezzotti, G. Rezza; Sassari: M.S. Mura, M. Mannazzu; Taranto: F. Resta, K. Loso; Torino: P. Caramello, A. Sinicco, M.L. Soranzo, G. Orofino, M. Sciandra, M. Bonasso; Varese: P.A. Grossi, C. Basilico; Verbania: A. Poggio, G. Bottari; Venezia: E. Raise, S. Pasquinucci; Vicenza: F. De Lalla, G. Tositti; London, UK: A. Cozzi Lepri.
Conflict of interest:
No information supplied
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
See “Acknowledgements” for the members of the ICONA Study Group
Rights and permissions
About this article
Cite this article
Merito, M., Pezzotti, P. & for the ICONA Study Group. Comparing costs and effectiveness of different starting points for highly active antiretroviral therapy in HIV-positive patients. Eur J Health Econ 7, 30–36 (2006). https://doi.org/10.1007/s10198-005-0327-9
Issue Date:
DOI: https://doi.org/10.1007/s10198-005-0327-9