Abstract
Background
Surgical site infections are important complications in orthopedic surgery. A mobile laminar air flow (LAF) screen could represent a useful addition to an operating room (OR) with conventional turbulent air ventilation (12.5 air changes/h), as it could decrease the bacterial count near the operating field. The purpose of this study was to evaluate LAF efficacy at reducing bacterial contamination in the surgical area during 34 total knee arthroplasties (TKAs).
Materials and methods
The additional unit was used in 17 operations; the LAF was positioned beside the operating table between two of the surgeons, with the air flow directed towards the surgical area (wound). The whole team wore conventional OR clothing and the correct hygiene procedures and rituals were used. Bacterial air contamination (CFU/m3) was evaluated in the wound area in 17 operations with the LAF unit and 17 without the LAF unit.
Results
The LAF unit reduced the mean bacterial count in the wound area from 23.5 CFU/m3 without the LAF to 3.5 CFU/m3 with the LAF (P < 0.0001), which is below the suggested limit for an OR with ultraclean laminar ventilation. There were no significant differences in the mean bacterial count in the instrument table area: 28.6 CFU/m3 were recorded with the LAF (N = 6) unit and 30.8 CFU/m3 (N = 6) without the LAF unit (P = 0.631). During six operations with LAF and six without LAF, particle counts were performed and the number of 0.5 μm particles was analyzed. The particle counts decreased significantly when the LAF unit was used (P = 0.003).
Conclusion
When a mobile LAF unit was added to the standard OR ventilation, bacterial contamination of the wound area significantly decreased to below the accepted level for an ultraclean OR, preventing SSI infections.
Similar content being viewed by others
Introduction
Surgical site infections (SSI) represent one of the most common complications in surgery. In particular, deep periprosthetic infections in orthopedic surgery constitute a disaster for both patient and surgeon.
Conservative estimates of infection rates average 1–2% for hip implants and 2–4% for knee implants [1, 2]. The number of joint replacements is expected to double in the next 20 years, and if the infection rate is not reduced, the incidence of infection will also double, yielding increased morbidity, hospital stays, and costs for the healthcare system [3, 4].
Periprosthetic infection rates have been shown to correlate with the number of airborne bacteria within 30 cm of the wound [5]. This is influenced by several factors relating to either the surgical environment (number of operating theater personnel, their clothing, type of ventilation system used) or the surgical procedure employed (approach, duration of exposure, use of a tourniquet).
The source of pathogens can be the patient (endogenous infection), the bacteria present in the OR air, instruments used, or the surgeon’s hands (exogenous infection). However, it is generally accepted that the main cause of surgical site infections (SSIs) after clean operations is bacterial contamination of the OR air, predominantly from contaminated skin scales shed by the surgical team, instruments used, or the surgeon’s hands [6–8].
Small numbers of organisms, including those of low pathogenicity, can cause orthopedic implant infections, and give rise to a considerable degree of morbidity and also mortality. It has been estimated that as few as ten colony forming units (CFU) are sufficient to cause deep infection in a prosthetic replacement arthroplasty. Bacteria that cause infection in the joint after total hip or knee replacement are inoculated into the wound at the time of insertion of the prosthesis [6, 9, 10].
The number of airborne bacteria in the OR is also dependent on the number of people present as well as their activities and behavior. Use of a ventilation system and appropriate personnel dress and discipline are therefore ways to reduce air contamination in the OR [1, 2, 4, 7–9, 11–26].
A laminar air flow ventilation system is recommended for an OR where orthopedic implant surgery is performed [9, 11]. Unfortunately, LAF systems are very expensive and complicated to install in a pre-existing OR. The introduction of a mobile LAF screen to complement the use of a standard ventilation system could be an effective but inexpensive way to decrease bacterial air contamination, as noted by Friberg et al. [7, 12] and Pasquarella et al. [8].
The aim of this study was to evaluate the efficacy of LAF at reducing bacterial contamination in the surgical area during orthopedic implant surgery in an OR with conventional turbulent air ventilation.
Materials and methods
Surgery
This study focused on 34 total knee replacements carried out over a period of two months.
All operations were performed in the same OR, early in the morning, and by the same surgeon; all cases received spinal anesthesia, tourniquet, and standard short-term antibiotic prophylaxis.
The additional LAF screen was used in 17 operations, while the remaining 17 operations were performed under ordinary conditions without the additional LAF screen.
Operating room
The experiments were performed within a standard OR (≈120 m3) equipped with a conventional turbulent ventilation system (with 12.5 air changes/h).
Mean thermohygrometric parameters were [11]: temperature, 20.6°C (0.1); relative humidity, 44.6% (3.1) (Table 1; Fig. 1).
Additional LAF screen
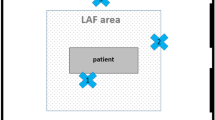
The additional LAF screen used in the study (Toul-400, Toul Meditech, Vasteras, Sweden) is a mobile unit that produces ultraclean exponential laminar air flow in a predefined area (the wound in our case). The additional mobile unit is a box with a fan and a HEPA filter (CAMFIL type, 99.997% particles >0.3 μm). The screen produces a laminar air flow of 0.5–0.7 m/s onto the wound and 0.4 m/s at the periphery. This exponential air flow prevents the entrainment of OR air outside the LAF [7, 8]. This air flow is turbulence-free and not impeded by the movements of the surgical team in the defined air flow area. The existing regular ventilation system does not affect the functioning of the unit. A camera assists in determining the direction of air flow, and an integrated sensor determines the correct distance from the surgical site for maximum effect. An integrated display allows the user to easily check and verify the setup (Toul Meditech data).
The LAF screen was positioned beside the operating table and between the two surgeons, with the air flow directed towards the surgical area, as shown in Fig. 1.
Surgical team
The surgical team consisted of a chief surgeon, an assistant surgeon, and two residents; there were also one chief anesthetist, one resident, one scrub nurse, one room nurse, and a technician. The team numbered between six and eight during all 34 operations.
Each member of the team wore conventional OR clothing during all operations: the surgeons, scrub nurse, and technician wore sterile nonwoven gowns, facemasks, surgical caps, and sterile gloves; the anesthetist and room nurse wore a woven OR uniform (shirt and trousers), facemask, surgical cap, and nonsterile gloves.
Sampling methods
Air contamination (in CFU/m3) was studied during all operations using a Biotest (Rockaway, NJ, USA) RCS Plus sampler (50 l/min) using Biotest HYCON agar strips TC-γ (γ-irradiated Total Count Tryptic Soy agar). The sampler was located 30 cm from the wound. Air counts were performed during 17 operations with the LAF screen and 17 operations without the LAF. Air quality on the instrument table was also investigated in six operations with and without the LAF unit. Sampling periods were always 20 min, and 1 m3 of air was sampled, as suggested by ISPESL guidelines [11] (Fig. 1; Table 2). The agar strips were incubated for 48 h at 37°C before counting the CFU, and the results were expressed in CFU/m3 (Table 3).
Particle counts were performed in the wound area during 12 operations (six with the LAF screen and six without) at the same time as the bacteria count using the Biotest (Dreieich, Germany) APC Plus (2.8 l/min). The sampling periods were again 20 min (Fig. 1; Table 2). Results were expressed in particles/m3, and ISO values for 0.5 μm particles were considered when interpretating the results [14] (Table 4).
Statistical analysis
SPSS (Statistical Package for Social Sciences) was used for statistical evaluations. The Mann–Whitney U test was used to establish significant differences between means. P ≤ 0.02 was regarded as significant.
Results
Mean bacterial air contamination in the wound area was 23.5 CFU/m3 under standard ventilation conditions; when the LAF unit was added to the standard ventilation, the mean bacterial count in the wound area decreased to 3.5 CFU/m3 (P < 0.0001) (Table 2), a reduction of about 85%, which is below the accepted limit (<10 CFU/m3) for ultraclean laminar ventilation [6, 9, 11, 13, 15].
In the instrument table area, the mean bacterial air contamination was almost the same whether or not the LAF unit was used (P = 0.631 = ns): 28.6 CFU/m3 with the LAF unit and 30.8 CFU/m3 without the LAF (Table 3).
When the LAF unit was used, there was a significant correlation between bacterial air contamination on the wound and instrument table (P = 0.0004); without the screen, no significant correlation was found (P = 0.361).
The mean numbers of 0.5 μm particles in the wound area and the instrument table area are shown in Table 4. Without the LAF screen, the mean value in the wound area was 970,533 particles/m3; upon adding the LAF screen, this mean was reduced to 17,361 particles/m3 (P = 0.003).
In the instrumental table area, the mean particles/m3 value ranged from 1,224,367 with the LAF unit to 1,380,181 without the LAF unit (P = 0.521 = ns).
No significant correlation was found between the particles/m3 values in the wound area and the instrument table area when the LAF unit was not used (P = 0.262); but when the LAF unit was added, a significant correlation was observed (P = 0.004).
Discussion
Many studies have demonstrated a correlation between airborne bacterial contamination and postoperative joint sepsis in arthroplasty surgery [5, 6, 9, 13, 22]. Correct rituals, surgical clothing, and the use of ultraclean laminar ventilation are strongly recommended in orthopedic implant surgery [7–9, 11–13, 18, 20–22] in order to reduce postoperative SSIs and respect the accepted limit of 10 CFU/m3 in the wound area for an ultraclean OR [14, 15].
We tested the efficacy of using an LAF unit in addition to a conventional air ventilation system in an implant surgery OR (12.5 air changes/h). We studied the effect of the screen on bacterial OR contamination during 34 total knee replacement operations. During the experiments, the surgical team respected the OR rituals and hygiene procedures and wore proper surgerical clothing. In our study, we used an active sampling method, as suggested in the ISPESL guidelines [11]. Bacterial sampling was performed in the wound area and the instrument table area to get an indication of bacterial contamination levels present under standard ventilation conditions and when the LAF screen is also used. We also decided to perform particle sampling in the same places and at the same time as the bacterial sampling during 12 operations (six with LAF and six without LAF), as an additional indicator of LAF unit efficacy.
The results suggested that the LAF screen is very effective at reducing bacterial contamination; the CFU/m3 value in the wound area was below the accepted limit for an ultraclean OR: the contamination in the wound area dropped from 23.5 CFU/m3 under standard ventilation conditions to 3.5 UFC/m3 when LAF was used, which is well below the limit of 10 CFU/m3 accepted for ultraclean laminar ventilation [6, 9, 13] and that of the UK (NHS) standard HTM 2025, which states that a limit of 20 CFU/m3 should not be exceeded in an OR with ultraclean laminar ventilation during surgical operations [11, 15]. This reduction in the CFU/m3 value in the wound area is statistically significant.
Bacterial contamination of the instrument table area did not change upon adding the LAF screen: it was 28.6 CFU/m3 with the LAF unit and 30.8 CFU/m3 without the LAF unit; no significant correlation between the level of contamination and whether LAF was functioning was observed, meaning that the influence of the LAF unit was limited to the focal area (in this case the wound area). The count of 0.5 μm particles in the wound area dropped from 970,533 particles/m3 when LAF was added to 17,361 particles/m3 when LAF was not working. This means that, for the wound area, the OR complied with ISO Class 8 standard conditions, and with ISO Class 6 standard conditions when the LAF unit was used. In the instrument table area, the particles count complied with ISO Class 8 conditions [14].
In conclusion, the prevention of infection is preferable to treatment in terms of both patient outcome and cost of treatment [4, 23–25]. Employing an additional ultraclean LAF unit reduced bacterial contamination and bacteria-carrying airborne particles in the surgical area (the wound) during total knee replacement operations. The CFU/m3 value in the wound area was reduced to below the limit suggested for implant surgery performed in an OR with ultraclean laminar ventilation.
A complete ultraclean LAF ventilation system is very expensive, and can sometimes be impossible to install in pre-existing premises without extensive rebuilding [3, 7]. Employing an additional LAF screen could be an interesting, effective, and inexpensive complement to OR standard ventilation when laminar air flow is required; in other words, for high-risk surgery (implant surgery, neurosurgery, transplant surgery), and in situations with insufficient ventilation or clothing facilities to reduce bacterial contamination and prevent SSI infections [7].
References
Hanssen AD, Rand JA (1999) Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect 48:111–122
Abudu A, Sivardeen KA, Grimer RJ et al (2002) The outcome of perioperative wound infection after total hip and knee arthroplasty. Int Orthop 26:40–43
Knobben BAS, Van Horn JR, Van der Mei HC, Busscher HJ (2006) Evaluation of measures to decrease intra-operative bacterial contamination in orthopaedic implant surgery. J Hosp Infect 62(2):174–180
Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R (2003) Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis 9:196–203
Lidwell OM, Lowbury EJL, White W, Blowers R, Stanley SJ, Lowe D (1983) Airborne contamination of wounds in joint replacement operations: the relationship to sepsis rates. J Hosp Infect 4:111–131
Whyte W, Hodgson R, Tinkler J (1982) The importance of airborne bacterial contamination of wounds. J Hosp Infect 3:123–135
Friberg S, Ardnor B, Lundholm R, Friberg B (2003) The addition of a mobile ultra-clean exponential laminar airflow screen to conventional operating room ventilation reduces bacterial contamination to operating box levels. J Hosp Infect 55:92–97
Pasquarella C, Sansebastiano GE, Ferretti S, Saccani E, Fanti M, Moscato U, Giannetti G, Fornia S, Cortellini P, Vitali P, Signorelli C (2007) A mobile laminar air flow unit to reduce air bacterial contamination at surgical area in a conventionally ventilated operating theatre. J Hosp Infect 66:313–319
Lidwell OM, Lowbury EJL, White W, Blowers R, Stanley SJ, Lowe D (1982) The effect of ultraclean air in operating rooms on deep sepsis in the joint after hip or knee replacement: a randomized study. Br Med J 285:10–14
Stocks GW, Self SD, Thompson B, Adame XA, O’Connor DP (2010) Predicting bacterial populations based on airborne particulates: a study performed in nonlaminar flow operating rooms during joint arthroplasty surgery. Am J Infect Control 38(3):199–204
ISPESL (2009) Guidelines on safety and hygiene standards in operating blocks. Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro, Rome
Friberg B, Lindgren M, Karlsson C, Bergstrom A, Friberg S (2002) Mobile zoned/exponential AF screen: a new concept in ultra-clean air technology for additional operating room ventilation. J Hosp Infect 50:286–292
Gosden PE, MacGowan AP, Bannister GC (1998) Importance of air quality in and related factors in the prevention of infection in orthopaedic implant surgery. J Hosp Infect 39:173–180
ISO (1999) UNI EN ISO 14644-1: 1999. Cleanrooms and associated controlled environments—part 1. Classification of air cleanliness. International Organization for Standardization, Geneva
National Health Service (2007) Health Technical Memorandum 2025: ventilation in healthcare premises. Department of Health, London
Dharan S, Pittet D (2002) Environmental controls in operating theatres. J Hosp Infect 51:79–84
Pereira ML, Tribess A (2005) A review of air distribution patterns in surgery rooms under infection control focus. Engenharia Térmica 2:113–121
Woodhead K, Taylor EW, Bannister G, Chesworth T, Hoffman P, Humphreys H (2002) Behaviours and rituals in the operating theatre. A report from the Hospital Infection Society Working Party on Infection Control in Operating Theatres. J Hosp Infect 51:241–255
Pasquarella C, Pitzurra O, Savino A (2000) The index of microbial air contamination. J Hosp Infect 46:241–256
Scheibel JH, Jensen I, Pederson S (1991) Bacterial contamination of air and surgical wounds during joint replacement operations. Comparison of two different types of staff clothing. J Hosp Infect 19:167–174
Lidwell OM, Elson RA, Lowbury EJ, Whyte W, Blowers R, Stanley SJ et al (1987) Ultraclean air and antibiotics for prevention of postoperative infection. A multicenter study of 8052 joint replacement operations. Acta Orthop Scand 58:4–13
Edmiston CE Jr, Seabrook GR, Cambria RA, Brown KR, Lewis BD, Sommers JR et al (2005) Molecular epidemiology of microbial contamination in the operating room environment: is there a risk of infection? Surgery 138:573–579
Bengtson S (1993) Prosthetic osteomyelitis with special reference to the knee: risks, treatment and costs. Ann Med 25:523–529
Sculco TP (1995) The economic impact of infected joint arthroplasty. Orthopedics 18:871–873
Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ (2002) The impact of surgical-site infections following orthopedic surgery at Community Hospital and a University Hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol 24(4):183–189
Evans RP (2011) Current concept for clean air anf total joint arthroplasty: laminar airflow and ultraviolet radiation: a systematic review. Clin Orthop Relat Res 469(4):945–953
Conflict of interest
None.
Ethical standards
The authors state that the study conforms to the Declaration of Helsinky as revised in 2008. All the patients provided informed consent to be involved in the study. No ethical commitee evaluation was requested since the research focussed on the environment of the Operating Room, not on the patients, who received a standard-of-care treatment.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sossai, D., Dagnino, G., Sanguineti, F. et al. Mobile laminar air flow screen for additional operating room ventilation: reduction of intraoperative bacterial contamination during total knee arthroplasty. J Orthopaed Traumatol 12, 207–211 (2011). https://doi.org/10.1007/s10195-011-0168-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10195-011-0168-5