Abstract
Introduction
The aim of this study was to show the clinical and pathological characteristics of anti-centromere-antibody (ACA)-seropositive Sjögren’s syndrome (SS) in two anti-human T-cell leukemia virus type I (HTLV-I)-seropositive patients.
Methods
One patient was an HTLV-I carrier whereas the other was diagnosed with HTLV-I-associated myelopathy (HAM). Background data including serum HTLV-I titers, viral loads, and cytokine profiles were recorded. Azocarmine with aniline blue (Azan)–Mallory staining and immunohistochemistry of the labial salivary glands (LSGs) and a muscle biopsy specimen from the HAM patient were performed.
Results
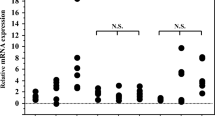
Serum transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α), and HTLV-I viral load were high in the HAM-SS patient compared with the HTLV-I carrier. Fibrous change in LSG was prominent in the HAM-SS patient. Although TGF-β expression was similar in the two patients, expression of HTLV-I-related proteins including p12, p28, group-specific antigen (GAG), and nuclear factor kappa-B (NF-κB) in the LSG were dominantly detected in the HAM-SS patient. Frequency of TGF-β staining in HTLV-I-seropositive SS patients without ACA, HTLV-I-seronegative SS patients with ACA, and HTLV-I-seronegative SS patients without ACA was lower than that of the previous two patients.
Conclusion
A high HTLV-I viral load in situ is supposed to promote the production of cytokines, especially TGF-β, resulting in the fibrous change of LSG in ACA-seropositive SS patients.




Similar content being viewed by others
Abbreviations
- ACA:
-
Anti-centromere antibody
- ANA:
-
Anti-nuclear antibody
- CSF:
-
Cerebrospinal fluid
- HAM:
-
HTLV-I-associated myelopathy
- HTLV-I:
-
Human T-cell leukemia virus type I
- IFN-γ:
-
Interferon gamma
- MNC:
-
Mononuclear cell
- LSG:
-
Labial salivary gland
- SS:
-
Sjögren’s syndrome
- TGF-β:
-
Transforming growth factor beta
- TNF-α:
-
Tumor necrosis factor alpha
References
Terada K, Katamine S, Eguchi K, Moriuchi R, Kita M, Shimada H, et al. Prevalence of serum and salivary antibodies to HTLV-1 in Sjögren’s syndrome. Lancet. 1994;344(8930):1116–9.
Nakamura H, Kawakami A, Eguchi K. Mechanisms of autoantibody production and the relationship between autoantibodies and the clinical manifestations in Sjögren’s syndrome. Transl Res. 2006;148:281–8.
Nakamura H, Eguchi K, Nakamura T, Mizokami A, Shirabe S, Kawakami A, et al. High prevalence of Sjögren’s syndrome in patients with HTLV-I associated myelopathy. Ann Rheum Dis. 1997;56:167–72.
Hida A, Imaizumi M, Sera N, Akahoshi M, Soda M, Maeda R, et al. Association of human T lymphotropic virus type I with Sjogren syndrome. Ann Rheum Dis. 2010;69:2056–7.
Nakamura H, Kawakami A, Tominaga M, Hida A, Yamasaki S, Migita K, et al. Relationship between Sjögren’s syndrome and human T-lymphotropic virus type I infection: follow-up study of 83 patients. J Lab Clin Med. 2000;135:139–44.
Nakamura H, Kawakami A, Hayashi T, Iwamoto N, Okada A, Tamai M, et al. Anti-centromere antibody-seropositive Sjögren’s syndrome differs from conventional subgroup in clinical and pathological study. BMC Musculoskelet Disord. 2010;11:140.
Katano K, Kawano M, Koni I, Sugai S, Muro Y. Clinical and laboratory features of anti-centromere antibody positive primary Sjögren’s syndrome. J Rheumatol. 2001;28:2238–44.
Hida A, Kawabe Y, Kawakami A, Migita K, Tominaga M, Nakamura H, et al. HTLV-I associated Sjögren’s syndrome is aetiologically distinct from anti-centromere antibodies positive Sjögren’s syndrome. Ann Rheum Dis. 1999;58:320–2.
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8.
Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjögren’s disease. J Clin Pathol. 1968;21:656–60.
Beroukas D, Hiscock J, Jonsson R, Waterman SA, Gordon TP. Subcellular distribution of aquaporin 5 in salivary glands in primary Sjögren’s syndrome. Lancet. 2001;358:1875–6.
Tsuboi H, Matsumoto I, Wakamatsu E, Nakamura Y, Iizuka M, Hayashi T, et al. New epitopes and function of anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren’s syndrome. Clin Exp Immunol. 2010;162:53–61.
Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–93.
Zhao T, Satou Y, Sugata K, Miyazato P, Green PL, Imamura T, et al. HTLV-1 bZIP factor enhances TGF-β signaling through p300 coactivator. Blood. 2011;118:1865–76.
Eguchi K, Matsuoka N, Ida H, Nakashima M, Sakai M, Sakito S, et al. Primary Sjögren’s syndrome with antibodies to HTLV-I: clinical and laboratory features. Ann Rheum Dis. 1992;51:769–76.
Santos SB, Porto AF, Muniz AL, Luna T, Nascimento MC, Guerreiro JB, et al. Modulation of T cell responses in HTLV-1 carriers and in patients with myelopathy associated with HTLV-1. Neuroimmunomodulation. 2006;13:145–51.
Hansen G, McIntire JJ, Yeung VP, Berry G, Thorbecke GJ, Chen L, et al. CD4(+) T helper cells engineered to produce latent TGF-beta1 reverse allergen-induced airway hyperreactivity and inflammation. J Clin Invest. 2000;105:61–70.
McCartney-Francis NL, Wahl SM. Dysregulation of IFN-gamma signaling pathways in the absence of TGF-beta 1. J Immunol. 2002;169:5941–7.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nakamura, H., Horai, Y., Tokuyama, A. et al. HTLV-I virological and histopathological analysis in two cases of anti-centromere-antibody-seropositive Sjögren’s syndrome. Mod Rheumatol 23, 133–139 (2013). https://doi.org/10.1007/s10165-012-0641-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-012-0641-x