Abstract
Unlike most other flower beetles, females of Dicronocephalus wallichii exhibit nesting behaviour. The female constructs a burrow in the soil, cuts dead plant leaves into small pieces to provision the nest, and then lays one egg inside the nest. Hatched larvae have been thought to feed on the nest materials prepared by their mothers, but the effects of pre-ovipositional care on larval performance have not been tested. The hatched larvae were found to stay in the nest for 15–30 days until they consumed the nest materials. We examined whether the presence of provisioned nests enhanced larval performance under both benign and food-stress conditions. With high-nutrient soil, larval survival rate and growth speed were not affected by the presence of provisioned nests. By contrast, with low-nutrient soil, mortality of the larvae was much higher in the absence than in the presence of provisioned nests. The growth speed of larvae with nests located in low-nutrient soil was as high as those reared in high-nutrient soil. These results indicate that females alleviate the food stress of larvae during their initial developmental stage by constructing provisioned nests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many animals provide care for their offspring, which increases their survival, growth, and quality (Trivers 1972; Clutton-Brock 1991; Royle et al. 2012). Parental care is known to have a greater positive effect on the fitness of offspring under unfavourable conditions such as high predation pressure, starvation, and inbreeding than under benign conditions (Royle et al. 2012; Chaboo et al. 2014; Pilakouta et al. 2015; Pike et al. 2016; but see Meunier and Kölliker 2012 and Kramer et al. 2017 for contrasting examples). Therefore, parental care has probably evolved as a way for parents to enhance their offspring’s fitness by offsetting the detrimental effects of a wide range of environmental stresses (Monteith et al. 2012; Chaboo et al. 2014; Pilakouta et al. 2015; Pike et al. 2016; Royle et al. 2016).
In insects, parental care takes many forms. Well-studied examples of parental care are post-ovipositional care including food provisioning to offspring (e.g. burying beetles Nicrophorus spp., leaf beetles Cassidinae, and subsocial bugs Cydnidae), offspring attendance (e.g. treehoppers Membracidae), and egg protection (e.g. water bugs, Belostomatidae) (Klug and Bonsall 2014; Santos et al. 2017). Parental care has been demonstrated to improve offspring performance in various insect species (Klug and Bonsall 2014; Santos et al. 2017). In addition, some species provide provision for their offspring before oviposition (pre-ovipositional care). For example, dung beetles (Scarabaeinae) and hunting wasps (Ammophila) leave food for offspring, and leaf-rolling beetles (Attelabidae) form protective structures for eggs and larvae (Klug and Bonsall 2014). Burying beetles Nicrophorus spp. bury vertebrate carcasses and roll them into balls, applying oral and anal antimicrobial secretions to their surfaces (Arce et al. 2012; Capodeanu-Nagler et al. 2016). However, the fitness benefits of pre-ovipositional care have been less studied than those of post-ovipositional care, with the exception of a few examples [e.g. Parastrachia japonensis (Filippi et al. 2000); Nicrophorus spp. (Capodeanu-Nägler et al. 2016; Trumbo 2017)].
Most flower beetles (Coleoptera: Scarabaeidae: Cetoniinae) do not show parental care. The females usually lay eggs in humus, and hatched larvae feed on organic matter and develop into adults by themselves. However, a previous study reported nesting behaviour by Dicronocephalus spp. (Šípek et al. 2008). Based on behavioural observations of Dicronocephalus adamsi, females first construct a burrow in the soil. Subsequently, females cut green or dead leaves of various plants into small pieces to fill the burrow. Females finally drag a small amount of soil into the burrow. Each burrow contains only one egg. Females then begin to construct another nest, and no further interaction between mother and offspring occurs. The construction of each nest lasts for several hours (Šípek et al. 2008). The nests constructed by Dicronocephalus females were hypothesised to benefit the development and survivorship of the larvae and eggs through physical protection from predators and increases in local temperature and humidity due to fermentation of leaves (Šípek et al. 2008). Furthermore, the nests may serve as a primary diet for newly hatched larvae based on the observation that the larvae ingest the nest materials (Šípek et al. 2008). However, the fitness effects of the nest materials on larval performance have not been tested.
Our main goal in this study is to clarify the function of the provisioned nests in a congener Dicronocephalus wallichii bourgoini Pouillaude 1914 (Fig. 1). Unlike D. adamsi, females of D. wallichii do not use green leaves; instead they show nesting behaviour by provisioning the dead leaves of various plant species (see Supplementary Material). Because high-quality food sources are generally patchy in the soil of forests, especially for the newly hatched insect larvae with limited mobility, we hypothesised that the pre-ovipositional maternal care of D. wallichii enhanced larval performance under harsh conditions (low-quality food sources). This study compared the survival rate and growth speed of larvae in the presence and absence of the nests under benign and food-stress conditions in the laboratory. Before conducting the experiments, we examined the duration that D. wallichii larvae stayed in the nests after hatching. Šípek et al. (2008) noted that the larvae stay in the nests for a few days, but quantitative data are lacking. Detailed information on the natural history of D. wallichii larvae is necessary to appropriately design experiments to test the effects of maternal care on larval performance.
Materials and methods
Study species
D. wallichii is distributed throughout Taiwan, Thailand, and southern China (Šípek et al. 2008). This species is univoltine (one generation per year) and adults emerge from April–June in Taiwan. They aggregate on tree sap sites and fruits to feed and mate (Kojima and Lin 2017). Females lay eggs in the nest. Hatched larvae develop into adults within 5–6 months. The enclosed adults overwinter in their cocoons, and emerge from the cocoons in the following spring (Šípek et al. 2008).
Insects and rearing conditions
Adult females were caught using an insect net in a lowland bamboo (Phyllostachys makinoi Hayata) forest in the district of Tamsui, Taipei, Taiwan (25°11′N, 121°29′E) in May 2016 and 2017. All experiments and insect rearing were conducted at 25 °C and a photoperiod of 12 h:12 h (light:dark).
Duration of larvae in the nest
We examined the duration that hatched larvae of D. wallichii stayed in the nest. Female adults were individually introduced into plastic containers (20 × 13-cm base, 13-cm height). The containers included fermented bark for rearing rhinoceros beetles (Dorcus Owner’s shop, Osaka, Japan) at a depth of 7 cm, which was covered with mixed dead leaves of bamboo and broadleaf trees (a mixture of Castanopsis spp. and Quercus spp.; approximately 80 g). We selected these leaves based on the results of a preliminary experiment of female preference (see Supporting Information). An insect jelly (Asagata-wide; SANKO, Japan) was provided. The insect jelly contains several kinds of sugars and amino acids, and is known to enhance the fecundity of sap-feeding beetles (Kojima 2015a). The nests were collected every 3 days until the females died. Cylindrical plastic containers (13-cm diameter × 7-cm height) filled with the fermented bark were prepared for the nests. The collected nests (expected to contain one egg) were individually and vertically buried in the centre of the cylindrical containers in the same way that they were constructed by females. It is unlikely that the process of nest collection and setting up affected larval performance because the larvae in the transplanted nests showed a high survival rate (> 80%) and developed as fast as those reported in a previous study (Šípek et al. 2008; see also Results). The eggs of this species are known to hatch approximately 10 days after being laid (Šípek et al. 2008; W. Kojima, personal observation). We gently dug the soil and located the larvae on the 25th, 30th, 35th or 40th day after we had collected and set up the nests. The body mass of the larvae was measured using an electric balance (ATX124; Shimadzu, Japan) to the nearest 0.01 mg. The sample size for each age class was 10–15 (n = 46 nests in total). If the larval head was within a circle with a radius of 2.5 cm from the centre of the cylindrical cup, the larva was defined as ‘inside the nest’. If the larval head was outside the circle, the larva was defined as ‘outside the nest’.
Effects of nests and dead leaves on larval performance
We examined the effects of nests and dead leaves (i.e. original nest material) on the survival and growth of hatched larvae under poorly and highly fermented soil environments. To collect larvae and nests, females were individually introduced into plastic containers (20 × 13-cm base, 13-cm height) with the fermented bark (7 cm in depth) and mixed dead leaves of bamboo and broadleaf trees (approximately 80 g). An insect jelly was provided. Nests were collected once every week. The eggs were carefully removed from the nests with tweezers to minimise the damage to the nests. The eggs were kept in 90-mL plastic containers with fermented bark. The eggs were checked every day and the hatched larvae were randomly assigned to three treatments: nest, leaves or bark only. For the nest treatment, 430-mL plastic containers were filled with the fermented bark (approximately 200 g) and a nest without an egg (5–7 g) was manually buried in the centre of the container in the same way as constructed by females. A newly hatched larva was put on the top of the nest. The larvae fed on the nests regardless of whether the nests were constructed by their own mothers. For the leaves treatment, the plastic containers were filled with a mixture of the fermented bark (approximately 200 g) and dead leaves (a mixture of bamboo and broadleaf tree leaves, approximately 40 g). This treatment was included in order to examine whether the processing of leaves by females (i.e. cutting into small pieces) had a positive effect on larval performance. A newly hatched larva was placed in the centre of the container. For the bark only treatment, the containers were filled with the fermented bark (approximately 200 g). A newly hatched larva was placed in the centre of the container. Because the amount of the fermented bark and dead leaves was much greater than the amount required by larvae during experimental periods (W. Kojima, personal observation), the larvae fed on them ad libitum. Twenty days later, we checked if the larvae were dead or alive and measured their body weight. The larvae of D. wallichii stay in the nests for at least 15–18 days after hatching (see “Results”). Thus, the experimental period (20 days) is sufficient to detect the effects of nest feeding. The number of larvae used was 16–18 for each treatment.
We also compared the larval performance among the three treatments using less-fermented soil. Because females drag some soil into the nests when they construct nests (Šípek et al. 2008), we collected nests using poorly fermented soil as substrate for this experiment. Females were individually introduced into the plastic containers (20 × 13-cm base, 13-cm height) with poorly fermented bark that was sold for stag beetle rearing (Dorcus Owner’s Shop) at a depth of 7 cm and mixed dead leaves of bamboo and broadleaf trees. The poorly fermented bark was made from oak sawdust and wheat bran, and it has been reported to be less nutritious for larvae of a saprophagous scarab beetle Trypoxylus dichotomus than the fermented bark (Kojima 2015b). An insect jelly was provided in the containers, and the nests were collected once a week. The eggs in the nests were carefully removed. We assigned newly hatched larvae to the three treatments shown above (nest, leaves or bark only) using the poorly fermented bark as substrate. The number of larvae used was 18–24 for each treatment.
A generalised linear model (GLM) with a binomial distribution and a logit link function was used for the mortality analysis. The explanatory variables were substrate conditions (i.e. poorly and highly fermented bark), and additionally provided food with three treatments (i.e. nests, leaves and bark only), and their interactions. A Tukey post hoc analysis was conducted for pairwise comparison if the interaction term was significant. For larvae body mass analysis, a significant difference in the variance of larval mass was found among treatments (see “Results”). Moreover, the sample from the poorly fermented bark-only treatment was excluded due to the small sample size (n = 1; see “Results”). Thus, the interaction between soil conditions and additional food provided was not included in the model. Instead, Mood’s median test was used for overall and pairwise comparison (Kasuya 2001). For pairwise comparison, the significance level was adjusted using the Holm procedure. All statistical analyses were conducted using R version 3.1.1 (R Development Core Team 2013).
Results
Nesting behaviour
Females of D. wallichii showed nesting behaviour by constructing a hole in the soil and dragging small pieces of dead leaves into it. They then laid one egg inside each nest. Females built on average 5.1 ± 1.1 SE (n = 8 females) nests within a week (see Supplementary Material). The wet mass of a nest was 5.89 ± 0.75 SE g (n = 7), and their height and diameter were approximately 5 and 2.5 cm, respectively (Fig. 1b). Pieces of torn leaves were loosely intertwined without obvious mucus. We carefully checked the substrate bark after the removal of nests, but found no eggs. This suggests that females always constructed nests for oviposition.
Duration of larvae in the nest
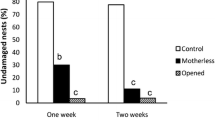
When we dug the bark on the 25th, 30th, 35th or 40th day after setting up the experiment, 28% of the containers (13/46) did not have a larva, probably because either larvae died or the nests originally did not contain an egg. Consequently, the sample size was smaller in some treatments (e.g. five out of ten containers had a larva 35–38 days later; Tables 1 and S1). The percentage of larvae in the nests began to decrease 25–28 days after eggs were laid; while all larvae (6/6) were in their nests at 25–28 days after eggs were laid, 15.4% of larvae (2/13) were found in the nests at 40–43 days after eggs were laid (Table 1). In many cases where larvae were outside the nests, almost all parts of the nests except for the top portion had been consumed by larvae and replaced by larval faeces.
The effects of nests and dead leaves on larval performance
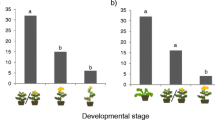
For mortality analysis, the interaction between Substrate conditions and Additional food provided was significant (likelihood ratio [LR] χ2 = 18.98, df = 2, P < 0.001, GLM; Fig. 2a; Table S2). Substrate conditions and Additional food provided were also significant (LR χ2 = 4.66, df = 1, P = 0.031 and LR χ2 = 25.00, df = 2, P < 0.001, respectively). When provided with only poorly fermented bark, 94% (17/18) of larvae died, and pairwise comparison showed that their mortality was significantly higher than that of any of the other five treatments (P < 0.001, GLM with Tukey method; Fig. 2a). There was no significant difference in mortality among the remaining five treatments (Fig. 2a).
a Mortality in the initial 20 days after larvae hatched, and b body mass of 20-day-old larvae in the larval performance test. Black lines indicate median values with upper and lower quartiles at the box edges; white dots mark potential outliers. Letters indicate significant difference in pairwise comparisons. For larval body mass, the single Bark only larva in the poorly fermented bark was excluded from statistical analysis because of the small sample size
The body mass of the one larva that survived in the treatment with only poorly fermented bark was 45 mg. This larva was excluded from statistical analysis. There was a significant difference in variance of larval mass among the five treatments (P = 0.041, Bartlett test). A significant difference in larval mass was found among the five treatments (P < 0.001, Mood’s median test; Fig. 2b). Pairwise comparison showed that the body mass of larvae provided with dead leaves in poorly fermented bark was significantly smaller than that of any of the other four treatments (P < 0.01, Mood’s median test with Holm method; Fig. 2b). There was no significant difference in larval mass among the remaining four treatments (Fig. 2b).
Discussion
The nesting behaviour of D. wallichii was very similar to that of its congener D. adamsi (Šípek et al. 2008). Neither of these species is a host-plant specialist in nest construction (Supporting Material; Šípek et al. 2008). However, while D. adamsi preferred fresh to dead leaves (Šípek et al. 2008), D. wallichii did not use fresh leaves (Supporting Material). Female preference for nest materials has probably diverged within this genus, but the ecological and evolutionary factors associated with this diversification are unknown due to the lack of information on the natural history of both species in the wild. However, the sample size (n = 8) might not have been sufficiently large to reveal female preference in this study, and our experiment did not test the preference for fresh leaves of broadleaf trees.
Eggs and their hatched larvae were present in the nests for 25–28 days after egg laying. From hatching, the larvae of D. wallichii stayed in the nests for at least 15–18 days. After that, they began to leave the nests. Eighty-five percent of larvae left their nests 30–33 days after hatching. Our results contradict a previous observation that the larvae of this species only stay in the nests for a few days (Šípek et al. 2008). However, the movement of larvae might be limited by the small container we used, and the duration of larvae in the nest could have been overestimated in our study. We also found a strong larval preference for nests in the choice tests (Supporting Material), indicating the importance of the nest materials as larval food. If the larvae are accidentally separated from their nests, they may return to the nest using unidentified chemical cues from the nests. When the larvae fully consume the nest materials, they probably start searching for alternative food such as dead leaves and organic debris found in the soil. The intraspecific variation in the period for which larvae stay in the nests may be related to the initial nest size, which was not assessed in this study.
We found that the feeding on nests enhances larval survival and growth rate in poorly fermented soil. Although other potential functions (e.g. protection from predators and increases in local humidity and temperature) were not tested, the nests likely serve as provision burrows. If nests or dead leaves were unavailable, almost all hatched larvae died. By contrast, with highly fermented soil, larvae showed a high survival rate and fast growth irrespective of the presence or absence of nests and dead leaves. Dead leaves or highly fermented organic matter are probably crucial for the survival of hatched larvae. We provided the 2nd instar larvae (approximately 20 days old, n = 10) with only poorly fermented bark, but all of them survived for at least 2 months (W. Kojima, personal observation). Thus, the importance of the nest materials likely decreases as larvae grow larger at later stages of larval development. Moreover, we found that the larvae provided with nests in poorly fermented soil grew as fast as larvae reared with highly fermented soil. This indicates that females alleviate the food stress of larvae during the initial developmental stage through constructing provisioned burrows. With poorly fermented soil, the speed of larval growth was slower when larvae were provided with original nest materials (i.e. dead leaves) than when they were provided with nests, although the amount of dead leaves provided (approximately 40 g) was sufficient and much larger than that of a nest (5–7 g). Large intact dead leaves are probably physically too hard for newly hatched larvae with small mandibles to eat, thus probably decrease larval feeding efficiency. Smaller pieces of dead leaves prepared by the females may be easier for the larvae to feed on than intact dead leaves. Furthermore, as has been reported in dung beetles (Estes et al. 2013; Schwab et al. 2016), D. wallichii females might inoculate beneficial microbes into the nests that help the digestion of food in the larval gut and/or enhance the nutritional quality of dead leaves through fermentation.
Constructing and provisioning nests probably entail costs to females. Females spend several hours constructing one nest (Šípek et al. 2008). We found that D. wallichii females laid less than 1 (0.73) egg/day on average, and the ovipositional rate is lower than that of other scarab beetles without nesting behaviour (Potter 1983; Brandhorst-Hubbard et al. 2001; Wenninger and Averill 2006; Kojima 2015a). The slower ovipositional rate in D. wallichii may be due to time- and energy-consuming nesting behaviour. Although we have no information on ovipositional sites of D. wallichii under natural conditions, suitable ovipositional sites with fermented soil are probably rare, patchy, and competitive among saprophagous animals. Therefore, it would be time- and energy-consuming for females to find highly fermented ovipositional substrate. In D. wallichii, the benefits via increased offspring performance in poorly fermented soil would likely outweigh the costs of constructing provisioned burrows.
In conclusion, we demonstrate that pre-ovipositional maternal care in D. wallichii enhances offspring survival and growth during their initial developmental stages under food-stress conditions. This study adds to the growing literature suggesting that parental care in animals has evolved as a means by which parents improve their offspring’s fitness under harsh conditions (Monteith et al. 2012; Chaboo et al. 2014; Pilakouta et al. 2015; Pike et al. 2016; Royle et al. 2016). Parental nesting behaviour using leaves has been reported in several scarab beetles [e.g. Lethrus apterus (Kosztolányi et al. 2015); Cephalodesmius armiger (López-Guerrero 1995); Dicronocephalus adamsi (Šípek et al. 2008)]. Further studies including phylogenetic comparative analyses would clarify the evolutionary driving forces for this type of maternal care.
Change history
31 December 2018
The article Ethological description of a fixed action pattern in a kissing bug (Triatominae): vision, gustation, proboscis extension
31 December 2018
The article Ethological description of a fixed action pattern in a kissing bug (Triatominae): vision, gustation, proboscis extension
31 December 2018
The article Ethological description of a fixed action pattern in a kissing bug (Triatominae): vision, gustation, proboscis extension
31 December 2018
The article Ethological description of a fixed action pattern in a kissing bug (Triatominae): vision, gustation, proboscis extension
References
Arce AN, Johnston PR, Smiseth PT, Rozen DE (2012) Mechanisms and fitness effects of antibacterial defences in a carrion beetle. J Evol Biol 25:930–937
Brandhorst-Hubbard JL, Flanders KL, Appel AG (2001) Oviposition site and food preference of the green June beetle (Coleoptera: Scarabaeidae). J Econ Entomol 94:628–633
Capodeanu-Nägler A, Keppner EM, Vogel H, Ayasse M, Eggert AK, Sakaluk SK, Steiger S (2016) From facultative to obligatory parental care: interspecific variation in offspring dependency on post-hatching care in burying beetles. Sci Rep 6:29323
Chaboo CS, Frieiro-Costa FA, Gómez-Zurita J, Westerduijn R (2014) Origins and diversification of subsociality in leaf beetles (Coleoptera: Chrysomelidae: Cassidinae: Chrysomelinae). J Nat Hist 48:2325–2367
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Estes AM, Hearn DJ, Snell-Rood EC, Feindler M, Feeser K, Abebe T, Hotopp JC, Moczek AP (2013) Brood ball-mediated transmission of microbiome members in the dung beetle, Onthophagus taurus (Coleoptera: Scarabaeidae). PLoS One 8:e79061
Filippi L, Hironaka M, Nomakuchi S, Tojo S (2000) Provisioned Parastrachia japonensis (Hemiptera: Cydnidae) nymphs gain access to food and protection from predators. Anim Behav 60:757–763
Kasuya E (2001) Mann-Whitney U-test when variances are unequal. Anim Behav 61:1247–1249
Klug H, Bonsall MB (2014) What are the benefits of parental care? The importance of parental effects on developmental rate. Ecol Evol 4:2330–2351
Kojima W (2015a) Variation in body size in the giant rhinoceros beetle Trypoxylus dichotomus is mediated by maternal effects on egg size. Ecol Entomol 40:420–427
Kojima W (2015b) Attraction to carbon dioxide from feeding resources and conspecific neighbours in larvae of the rhinoceros beetle Trypoxylus dichotomus. PLoS One 10:e0141733
Kojima W, Lin CP (2017) It takes two to tango: functional roles, sexual selection and allometry of multiple male weapons in the flower beetle Dicronocephalus wallichii bourgoini. Biol J Linn Soc 121:514–529
Kosztolányi A, Nagy N, Kovács T, Barta Z (2015) Predominant female care in the beetle Lethrus apterus with supposedly biparental care. Entomol Sci 18:292–294
Kramer J, Körner M, Diehl J, Scheiner C, Yüksel-Dadak A, Christl T, Kohlmeier P, Meunier J (2017) When earwig mothers do not care to share: parent-offspring competition and the evolution of family life. Funct Ecol 31:2098–2107
López-Guerrero Y (1995) Development and histology of the ovary in Cephalodesmius armiger Westwood (Coleoptera: Scarabaeidae, Scarabaeinae). Coleopt Bull 49:332–342
Meunier J, Kölliker M (2012) When it is costly to have a caring mother: food limitation erases the benefits of parental care in earwigs. Biol Lett 8:547–550
Monteith KM, Andrews C, Smiseth PT (2012) Post-hatching parental care masks the effects of egg size on offspring fitness: a removal experiment on burying beetles. J Evol Biol 25:1815–1822
Pike DA, Clark RW, Manica A, Tseng HY, Hsu JY, Huang WS (2016) Surf and turf: predation by egg-eating snakes has led to the evolution of parental care in a terrestrial lizard. Sci Rep 6:22207
Pilakouta N, Jamieson S, Moorad JA, Smiseth PT (2015) Parental care buffers against inbreeding depression in burying beetles. Proc Natl Acad Sci USA 112:8031–8035
Potter DA (1983) Effect of soil moisture on oviposition, water absorption, and survival of southern masked chafer (Coleoptera: Scarabaeidae) eggs. Environ Entomol 12:1223–1227
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Royle NJ, Smiseth PT, Kölliker M (2012) The evolution of prental care. Oxford University Press, Oxford
Royle NJ, Alonzo SH, Moore AJ (2016) Co-evolution, conflict and complexity: what have we learned about the evolution of parental care behaviours? Curr Opin Behav Sci 12:30–36
Santos ES, Bueno PP, Gilbert JD, Machado G (2017) Macroecology of parental care in arthropods: higher mortality risk leads to higher benefits of offspring protection in tropical climates. Biol Rev 92:1688–1701
Schwab DB, Riggs HE, Newton IL, Moczek AP (2016) Developmental and ecological benefits of the maternally transmitted microbiota in a dung beetle. Am Nat 188:679–692
Šípek P, Král D, Jahn O (2008) Description of the larvae of Dicronocephalus wallichi bourgoini Coleoptera: Scarabaeidae: Cetoniinae) with observations on nesting behavior and life cycle of two Dicronocephalus species under laboratory conditions. Ann Soc Entomol Fr 44:409–417
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine, Chicago, pp 136–179
Trumbo ST (2017) Feeding upon and preserving a carcass: the function of prehatch parental care in a burying beetle. Anim Behav 130:241–249
Wenninger EJ, Averill AL (2006) Effects of delayed mating on reproductive output of female oriental beetle Anomala orientalis (Coleoptera: Scarabaeidae). Agric Forest Entomol 8:221–231
Acknowledgements
We are very grateful to Dr G. Machado and two anonymous reviewers for valuable comments on the manuscript.
Funding
This study was supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad to Wataru Kojima and research grants of the Ministry of Science and Technology (MOST, 103-2311-B-029-001-MY3 and 104-2621-B-003-002-MY3) to Chung-Ping Lin.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kojima, W., Lin, CP. Pre-ovipositional maternal care alleviates food stress of offspring in the flower beetle Dicronocephalus wallichii. J Ethol 36, 135–141 (2018). https://doi.org/10.1007/s10164-018-0544-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-018-0544-1