Abstract
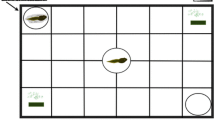
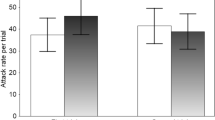
Tadpoles of Sphaerotheca breviceps detect food and predators using waterborne chemical cues (odours), which evoke appropriate behaviour. The present study aimed to determine whether they are capable of resolving the conflict between foraging choice and predator avoidance on encountering both food and predatory chemical cues together. Experiments were designed using an association choice apparatus in which prey tadpoles were given a choice to move themselves close to or away from a given stimulus (food or a predator). Food odours were either strong or weak. Tadpoles of Hoplobatrachus tigerinus (n = 4) were placed in an open-ended mesh cage wrapped in a cheese cloth that provided predator cues. The time spent by the test tadpoles in the predator’s zone or away from it and the time spent feeding were recorded for 10 min (n = 25 trials). When food odours were weak, the prey tadpoles avoided predators and spent significantly more time in the predator-free zone. In contrast, when food odours were strong, they foraged equally in the two zones of the test apparatus regardless of the presence or absence of predators. These findings confirm the inherent ability of S. breviceps tadpoles to distinguish between food and predators, and indicate that strong food odours mask predation risk. We suggest that foraging theory and the predation risk allocation hypothesis should include factors that mislead prey during predation risk assessment and the evocation of defence behaviours.


Similar content being viewed by others
References
Benard MF (2004) Predator-induced phenotypic plasticity in organisms with complex life histories. Annu Rev Ecol Evol Syst 35:651–673
Bridges CM (2002) Tadpoles balance foraging and predator avoidance: effects of predation pond drying and hunger. J Herpetol 36:627–634
Duellman WE, Trueb L (1994) Biology of amphibians, 2nd edn. Johns Hopkins University Press, Baltimore
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hoff KVS, Blaustein AR, McDiarmid RW, Altig R (1999) Behavior: interactions and their consequences. In: McDiarmid RW, Altig R (eds) Tadpoles: biology of anuran larvae. University of Chicago Press, Chicago, pp 215–239
Horat P, Semlitsch RD (1994) Effects of predation risk and hunger on the behavior of two species of tadpoles. Behav Ecol Sociobiol 34:393–401
Hossie TJ, Murray DL (2010) You can’t run but you can hide: refuge use in frog tadpoles elicits density-dependent predation by dragonfly larvae. Oecologia 163:395–404
Kiesecker JM, Chivers DP, Blaustein AR (1996) The use of chemical cues in predator recognition by western toad tadpoles. Anim Behav 52:1237–1245
Laurila A, Kujasalo J (1999) Habitat duration, predation risk and phenotypic plasticity in common frog (Rana temporaria) tadpoles. J Anim Ecol 68:1123–1132
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Milinski M, Heller R (1978) Influence of predator on the optimal foraging behaviour of sticklebacks (Gasterosteus aculeaatus L.). Nature 275:642–644
Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA (2005) Ecological consequences of phenotypic plasticity. Trends Ecol Evol 20:685–692
Mogali SM, Saidapur SK, Shanbhag BA (2011a) Levels of predation modulates antipredator defense behavior and metamorphic traits in the toad, Bufo melanostictus. J Herpetol 45:428–431
Mogali SM, Saidapur SK, Shanbhag BA (2011b) Receding water levels hasten metamorphosis in the frog, Sphaerotheca breviceps (Schneider, 1799): a laboratory study. Curr Sci 101:1219–1222
Mogali SM, Saidapur SK, Shanbhag BA (2012) Tadpoles of the bronze frog (Rana temporalis) assess predation risk before evoking antipredator defense behavior. J Ethol 30:379–386
Nystrom P, Abjornsson K (2000) Effects of fish chemical cues on the interactions between tadpoles and crayfish. Oikos 88:181–190
Saidapur SK (2001) Behavioral ecology of anuran tadpoles: the Indian scenario. Proc Indian Natl Sci Acad (PINSA) B67:311–322
Saidapur SK, Veeranagoudar DK, Hiragond NC, Shanbhag BA (2009) Mechanism of predator–prey detection and behavioral responses in some anuran tadpoles. Chemoecology 19:21–28
Schoeppner NM, Relyea RA (2005) Damage, digestion and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512
Sharma SS, Veeranagoudar DK, Shanbhag BA, Saidapur SK (2008) Activity of Sphaerotheca breviceps tadpoles in response to chemical cues of the predaceous Hoplobatrachus tigerinus tadpoles. J Ethol 26:303–307
Skelly DK (1994) Activity level and the susceptibility of anuran larvae to predation. Anim Behav 47:465–468
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71:2312–2322
Stauffer H, Semlitsch RD (1993) Effects of visual, chemical and tactile cues of fish on the behavioral responses of tadpoles. Anim Behav 46:355–364
Sugur HS, Mulla GS, Purohit IR, Shanbhag BA, Saidapur SK (2008) Sensory basis of food perception in tadpoles of the frog, Sphaerotheca breviceps. Curr Sci 95:1743–1746
Toledo LF, Ribeiro RS, Haddad CFB (2007) Anurans as prey: an exploratory analysis and size relationships between predators and their prey. J Zool 271:170–177
Veeranagoudar DK, Shanbhag BA, Saidapur SK (2004) Foraging behavior in tadpoles of the bronze frog Rana temporalis: experimental evidence for the ideal free distribution. J Biosci 29:201–207
Zug GR, Vitt LJ, Caldwell JP (2001) Herpetology: an introductory biology of amphibians and reptiles, 2nd edn. Academic, San Diego, pp 275–298
Acknowledgments
This work was supported by a grant from the Department of Science and Technology (SP/SO/AS-38/2009), New Delhi, awarded to BAS and SKS. They also thank the Indian National Science Academy, New Delhi for support. SMM thanks UGC New Delhi for a D.S. Kothari Postdoctoral Fellowship. All work reported herein was conducted in accordance with ethical guidelines laid down by CPCSEA, New Delhi, India (registration no. 639/02/a/CPCSEA).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mogali, S.M., Shanbhag, B.A. & Saidapur, S.K. Strong food odours mask predation risk and affect evocation of defence behaviours in the tadpoles of Sphaerotheca breviceps . J Ethol 33, 41–46 (2015). https://doi.org/10.1007/s10164-014-0415-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-014-0415-3