Abstract
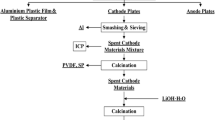
In this paper, a process route for the recovery and resynthesis of spent ternary lithium battery cathode materials was proposed, combined with the recovery process of hydrometallurgy and pyrometallurgy. The results showed that LiNi0.6Co0.2Mn0.2O2 (NCM622) could be completely decomposed into a mixture of Co, Ni, MnO and Li2CO3 by carbothermal reduction roasting process at 650 °C, graphite content of 14% and roasting time of 2 h. The roasted product was subjected to carbonation water leaching at a liquid–solid ratio of 30 ml/g at 20 °C for 60 min to achieve a lithium-leaching rate of 93.33%. For the obtained water-leaching residue, the leaching rate of Mn, Co and Ni could reach 96.63%, 95.57% and 94.22%, respectively, by acid leaching with 3 mol/l H2SO4 at 10 ml/g liquid–solid ratio. Subsequently, the mixed sulfate solution was used to prepare the precursor by co-precipitation method, and then a ternary cathode material could be synthesized by solid-phase sintering with Li2CO3. The electrochemical test of the resynthesized cathode material was carried out. The initial discharge specific capacity was 175.2 mAh/g, and the capacity retention rate was 91.60% after 100 cycles. The excellent performance shown by the cathode material verifies the feasibility of this recycling method.







Similar content being viewed by others
References
Fan E, Li L, Wang Z, Lin J, Huang Y, Yao Y, Chen R, Wu F (2020) Sustainable recycling technology for Li-ion batteries and beyond: challenges and future prospects. Chem Rev 120(14):7020–7063. https://doi.org/10.1021/acs.chemrev.9b00535
Amici J, Asinari P, Ayerbe E, Barboux P, Bayle-Guillemaud P, Behm RJ, Berecibar M, Berg E, Bhowmik A, Bodoardo S, Castelli IE, Cekic-Laskovic I, Christensen R, Clark S, Diehm R, Dominko R, Fichtner M, Franco AA, Grimaud A, Guillet N, Hahlin M, Hartmann S, Heiries V, Hermansson K, Heuer A, Jana S, Jabbour L, Kallo J, Latz A, Lorrmann H, Martin Løvvik O, Lyonnard S, Meeus M, Paillard E, Perraud S, Placke T, Punckt C, Raccurt O, Ruhland J, Sheridan E, Stein H, Tarascon J-M, Trapp V, Vegge T, Weil M, Wenzel W, Winter M, Wolf A (2022) A roadmap for transforming research to invent the batteries of the future designed within the European Large Scale Research Initiative BATTERY 2030+. Adv Energy Mater 12(17):2102785–2102793. https://doi.org/10.1002/aenm.202102785
Hu S, He S, Jiang X, Wu M, Wang P, Li L (2021) Forecast and suggestions on the demand of lithium, cobalt, nickel and manganese resources in China’s new energy automobile industry. IOP Conf Ser: Earth Environ Sci 769(4):042018. https://doi.org/10.1088/1755-1315/769/4/042018
Guo Y, Liao X, Huang P, Lou P, Su Y, Hong X, Han Q, Yu R, Cao Y-C, Chen S (2021) High reversibility of layered oxide cathode enabled by direct re-generation. Energy Storage Mater 43:348–357. https://doi.org/10.1016/j.ensm.2021.09.016
Guo Y, Li F, Zhu H, Li G, Huang J, He W (2016) Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Manage 51:227–233. https://doi.org/10.1016/j.wasman.2015.11.036
Yano J, Xu G, Liu H, Toyoguchi T, Iwasawa H, Sakai S (2019) Resource and toxic characterization in end-of-life vehicles through dismantling survey. J Mater Cycles Waste Manage 21(6):1488–1504. https://doi.org/10.1007/s10163-019-00902-9
Tong L (2016) New energy vehicle waste lithium battery environmental hazards and treatment methods. Resour Econ Environ 8:67–71. https://doi.org/10.16317/j.cnki.12-1377/x.2016.08.054
Zhang W, Xu C, He W, Li G, Huang J (2018) A review on management of spent lithium ion batteries and strategy for resource recycling of all components from them. Waste Manage Res 36(2):99–112. https://doi.org/10.1177/0734242X17744655
Barik SP, Prabaharan G, Kumar B (2016) An innovative approach to recover the metal values from spent lithium-ion batteries. Waste Manage 51:222–226. https://doi.org/10.1016/j.wasman.2015.11.004
Takacova Z, Havlik T, Kukurugya F, Orac D (2016) Cobalt and lithium recovery from active mass of spent Li-ion batteries: theoretical and experimental approach. Hydrometallurgy 163:9–17. https://doi.org/10.1016/j.hydromet.2016.03.007
Chu S, Cui Y, Liu N (2017) The path towards sustainable energy. Nat Mater 16(1):16–22. https://doi.org/10.1038/nmat4834
Hu J, Zhang J, Li H, Chen Y, Wang C (2017) A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J Power Sources 351:192–199. https://doi.org/10.1016/j.jpowsour.2017.03.093
Joulié M, Laucournet R, Billy E (2014) Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J Power Sources 247:551–555. https://doi.org/10.1016/j.jpowsour.2013.08.128
Tang H, Tan F, Dai X, Qiao Y, Zheng X (2020) Comprehensive recovery of mixed spent of LiNixCoyMn(1–x−y)O2 and LiFePO4. J Mater Cycles Waste Manage 22(6):1734–1743. https://doi.org/10.1007/s10163-020-01059-6
Wang W-Y, Yen CH, Lin J-L, Xu R-B (2019) Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J Mater Cycles Waste Manage 21(2):300–307. https://doi.org/10.1007/s10163-018-0790-x
Gu F, Guo J, Yao X, Summers PA, Widijatmoko SD, Hall P (2017) An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China. J Clean Prod 161:765–780. https://doi.org/10.1016/j.jclepro.2017.05.181
Sun L, Qiu K (2011) Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J Hazard Mater 194:378–384. https://doi.org/10.1016/j.jhazmat.2011.07.114
Massé RC, Uchaker E, Cao G (2015) Beyond Li-ion: Electrode materials for sodium- and magnesium-ion batteries. Sci China Mater 58(9):715–766. https://doi.org/10.1007/s40843-015-0084-8
Huang B, Yang J, Li Y, Xiao S, Chen Q (2018) Carbon encapsulated Sn-Co alloy: a stabilized tin-based material for sodium storage. Mater Lett 210:321–324. https://doi.org/10.1016/j.matlet.2017.09.055
Zhu X, Xiao J, Mao Q, Zhang Z, You Z, Tang L, Zhong Q (2022) A promising regeneration of waste carbon residue from spent Lithium-ion batteries via low-temperature fluorination roasting and water leaching. Chem Eng J 430:132703. https://doi.org/10.1016/j.cej.2021.132703
Li J, Wang G, Xu Z (2016) Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J Hazard Mater 302:97–104. https://doi.org/10.1016/j.jhazmat.2015.09.050
Xiao J, Li J, Xu Z (2017) Novel approach for in situ recovery of lithium carbonate from spent lithium ion batteries using vacuum metallurgy. Environ Sci Technol 51(20):11960–11966. https://doi.org/10.1021/acs.est.7b02561
Lombardo G, Ebin B, St J, Foreman MR, Steenari B-M, Petranikova M (2019) Chemical transformations in Li-ion battery electrode materials by carbothermic reduction. ACS Sustain Chem Eng 7(16):13668–13679. https://doi.org/10.1021/acssuschemeng.8b06540
Tang Y, Xie H, Zhang B, Chen X, Zhao Z, Qu J, Xing P, Yin H (2019) Recovery and regeneration of LiCoO2-based spent lithium-ion batteries by a carbothermic reduction vacuum pyrolysis approach: controlling the recovery of CoO or Co. Waste Manage 97:140–148. https://doi.org/10.1016/j.wasman.2019.08.004
Yao L, Feng Y, Xi G (2015) A new method for the synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries. RSC Adv 5(55):44107–44114. https://doi.org/10.1039/C4RA16390G
Yang Y, Huang G, Xie M, Xu S, He Y (2016) Synthesis and performance of spherical LiNixCoyMn1-x-yO2 regenerated from nickel and cobalt scraps. Hydrometallurgy 165:358–369. https://doi.org/10.1016/j.hydromet.2015.11.015
Mao J, Li J, Xu Z (2018) Coupling reactions and collapsing model in the roasting process of recycling metals from LiCoO2 batteries. J Clean Prod 205:923–929. https://doi.org/10.1016/j.jclepro.2018.09.098
Guo X, Cao X, Huang G, Tian Q, Sun H (2017) Recovery of lithium from the effluent obtained in the process of spent lithium-ion batteries recycling. J Environ Manage 198:84–89. https://doi.org/10.1016/j.jenvman.2017.04.062
Sasai R, Fujimura T, Uesugi R, Aketo T, Komatsu K (2023) Eco-friendly recovery process of lithium from reduction roasting residue powder based on hydrothermal extraction. J Mater Cycles Waste Manage 25(1):389–395. https://doi.org/10.1007/s10163-022-01546-y
Wang L, He XM, Gao J, Li JJ, Jiang CY (2018) Manufacturing method for cathode materials of Li-ion batteries. Energy Storage Sci Technol 7(5):888–896
Xu QJ, Zhou LZ, Liu MS, Pan HT, Deng XQ (2012) Research progress in cathode material of Li-Ni-Co-Mn-O for lithium ion battery. J Shanghai Univ Electr Power 28(2):143–148
Cho TH, Park SM, Yoshio M, Hirai T, Hideshima Y (2005) Effect of synthesis condition on the structural and electrochemical properties of Li[Ni1/3Mn1/3Co1/3]O2 prepared by carbonate co-precipitation method. J Power Sources 142(1):306–312. https://doi.org/10.1016/j.jpowsour.2004.10.016
Zhang C, Yang P, Dai X, Xiong X, Zhan J, Zhang Y (2009) Synthesis of LiNi1/3Co1/3Mn1/3O2 cathode material via oxalate precursor. Trans Nonferr Met Soc China 19(3):635–641. https://doi.org/10.1016/S1003-6326(08)60325-8
Yang Y, Xu SM, Weng YQ, Huang GY, Li LY (2013) Preparation and characterization of xLi2MnO3·(1-x)Li(Ni1/3Co1/3Mn1/3)O2(x=0.2, 0.4, 0.6) cathode materials synthesized by hydroxide co-precipitation method. J Funct Mater 44(19):2878–2881+2887
Zhang Y, Wang W, Fang Q, Xu S (2020) improved recovery of valuable metals from spent lithium-ion batteries by efficient reduction roasting and facile acid leaching. Waste Manage 102:847–855. https://doi.org/10.1016/j.wasman.2019.11.045
Liu P, Xiao L, Tang Y, Chen Y, Ye L, Zhu Y (2019) Study on the reduction roasting of spent LiNixCoyMnzO2 lithium-ion battery cathode materials. J Therm Anal Calorim 136:1323–1332. https://doi.org/10.1007/s10973-018-7732-7
Cherkashinin G, Motzko M, Schulz N, Späth T, Jaegermann W (2015) Electron spectroscopy study of Li[Ni Co, Mn]O2/electrolyte interface: electronic structure, interface composition, and device implications. Chem Mater 27(8):2875–2887. https://doi.org/10.1021/cm5047534
Winter M, Barnett B, Xu K (2018) Before Li ion batteries. Chem Rev 118(23):11433–11456. https://doi.org/10.1021/acs.chemrev.8b00422
Duan J, Hu G, Cao Y, Tan C, Wu C, Du K, Peng Z (2016) Enhanced electrochemical performance and storage property of LiNi0.815Co0.15Al0.035O2 via Al gradient doping. J Power Sources 326:322–330. https://doi.org/10.1016/j.jpowsour.2016.07.008
Acknowledgements
This work was supported by the National Key R&D Program of China (2019YFC1905703).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., You, X., She, X. et al. Research on the process of carbon thermal reduction for recovery and resynthesis of LiNi0.6Co0.2Mn0.2O2. J Mater Cycles Waste Manag 26, 346–359 (2024). https://doi.org/10.1007/s10163-023-01835-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01835-0