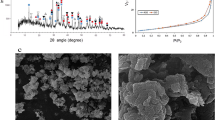
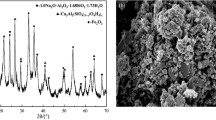
Abstract
Red mud contains excessive Na+, which seriously damages acidity and alkalinity of surrounding soil and groundwater. Therefore, the occurrence form and the leaching characteristics of the alkaline components in red mud under different pH conditions were studied by X-ray diffraction (XRD), ion chromatography and titration test, et al., and the solidification mechanism of the hydration products of red mud-based geo-polymer cementitious materials (RMC) and Na+ solidify agent, such as 732 H-type cation exchange resin, sodium humate, chitosan, and hydroxyapatite on the alkaline components, was explored through ICP-OES, XRD, SEM–EDS, et al. The results show that: (1) The alkaline components in the red mud are mainly Na components, and the soluble Na components are mainly in the form of NaHCO3 and Na2CO3, accounting for 8.87 wt%, 6.35 wt%. (2) With the increase of curing age, the leaching concentration of Na+ in RMC gradually decreases, and the solidify rate of Na+ gradually increases. When the curing age is 120 d, the solidify rate of Na+ tends to be stable, reaching 63.64%, which solidifies Na+ through self-cementation and adsorption. (3) The optimal Na+ solidify agents are chitosan with a content of 3%. When the curing age is 28 days, the solidify rate of Na+ reaches 73.76%. (4) The solidify rate of Na+ can be improved by forming adsorption chemical bonds through electron transfer, exchange, or coownership between Na+ and hydration products. The synergistic effect of the polymer gel and Na+ solidify agent improved the mechanics and the solidify rate of Na+ in RMC.



















Similar content being viewed by others
Data and code availability
Some or all data, models, or code that supports the findings of this study is available from the corresponding author upon reasonable request. Some data are openly available as part of a Master’s thesis, published openly by Shandong University.
References
Ren H, Tang Y, Shi W, Chen F, Yusong Xu (2019) Red mud modified with graphene oxide for enhanced visible-light-driven photocatalytic performance towards the degradation of antibiotics. New J Chem 43(48):19172–19179. https://doi.org/10.1039/C9NJ04697F
Li S, Zhang J, Li Z, Liu C, Chen J (2021) Feasibility study on grouting material prepared from red mud and metallurgical wastewater based on synergistic theory. J Hazard Mater 407:124358. https://doi.org/10.1016/j.jhazmat.2020.124358
Li Y, Min X, Ke Y, Chai L, Shi M, Tang C, Wang Q, Liang Y, Lei J, Liu D (2018) Utilization of red mud and Pb/Zn smelter waste for the synthesis of a red mud-based cementitious material. J Hazard Mater 344:343–349. https://doi.org/10.1016/j.jhazmat.2017.10.046
Wen Z, Ma S, Zheng S, Zhang Yi, Liang Y (2016) Assessment of environmental risk for red mud storage facility in China: a case study in Shandong province. Environ Sci Pollut Res Int 23(11):11193–11208. https://doi.org/10.1007/s11356-016-6243-y
Li S, Zhang J, Li Z, Gao Y, Liu C (2021) Feasibility study of red mud-blast furnace slag based geopolymeric grouting material: effect of superplasticizers. Constr Build Mater 267:120910. https://doi.org/10.1016/j.conbuildmat.2020.120910
Wang Y, Zhang T-a, Lyu G, Guo F, Zhang W, Zhang Y (2018) Recovery of alkali and alumina from bauxite residue (red mud) and complete reuse of the treated residue. J Cleaner Prod 188:456–465. https://doi.org/10.1016/j.jclepro.2018.04.009
Oprčkal P, Mladenovič A, Zupančič N, Ščančar J, Milačič R, Zalar Serjun V (2020) Remediation of contaminated soil by red mud and paper ash. J Cleaner Prod 256:120440. https://doi.org/10.1016/j.jclepro.2020.120440
Liu R, Poon C (2016) Utilization of red mud derived from bauxite in self-compacting concrete. J Cleaner Prod 112(3):384–391. https://doi.org/10.1016/j.jclepro.2015.09.049
Newson T, Dyer T, Adam C, Sharp S (2006) Effect of structure on the geotechnical properties of bauxite residue. J Geotech Geo-environ 132(2):143–151. https://doi.org/10.1061/(ASCE)1090-0241(2006)132:2(143)
Wang Z, Wang Y, Libo Wu, Aixiang Wu, Ruan Z, Zhang M, Zhao R (2022) Effective reuse of red mud as supplementary material in cemented paste backfill: durability and environmental impact. Constr Build Mater 328:127002. https://doi.org/10.1016/j.conbuildmat.2022.127002
Zhang Na, Liu X, Sun H, Li L (2011) Evaluation of blends bauxite-calcination-method red mud with other industrial wastes as a cementitious material: properties and hydration characteristics. J Hazard Mater 185(1):329–335. https://doi.org/10.1016/j.jhazmat.2010.09.038
Senff L, Modolo RCE, Santos Silva A, Ferreira VM, Hotza D, Labrincha JA (2014) Influence of red mud addition on rheological behavior and hardened properties of mortars. Constr Build Mater 65:84–91. https://doi.org/10.1016/j.conbuildmat.2014.04.104
Chen S, Zhaowen Du, Zhang Z, Yin D, Feng F, Ma J (2020) Effects of red mud additions on gangue-cemented paste backfill properties. Powder Technol 367:833–840. https://doi.org/10.1016/j.powtec.2020.03.055
Li Z, Hannian Gu, Hong B, Wang N, Chen M (2022) An innovative process for dealkalization of red mud using leachate from Mn-containing waste. J Environ Chem Eng 10(2):107222. https://doi.org/10.1016/j.jece.2022.107222
Sun Z, Tang Q, Xakalashe BS, Fan X, MinGana XC, Ji Z, Huang X, Friedrich B (2022) Mechanical and environmental characteristics of red mud geopolymers. Constr Build Mater 321:125564. https://doi.org/10.1016/j.conbuildmat.2021.125564
Liang X, Ji Y (2021) Experimental study on durability of red mud-blast furnace slag geopolymer mortar. Constr Build Mater 267:120942. https://doi.org/10.1016/j.conbuildmat.2020.120942
Shaker Qaidi MA, Bassam Tayeh A, Haytham Isleem F, de Afonso Azevedo RG, Ahmed HU, Emad W (2022) Sustainable utilization of red mud waste (bauxite residue) and slag for the production of geopolymer composites: a review. Case Stud Constr Mater 16:e00994. https://doi.org/10.1016/j.cscm.2022.e00994
Ye N, Chen Ye, Yang J, Lianga S, Yong Hu, Jingping Hu, Zhu S, Fan W, Xiao Bo (2017) Transformations of Na, Al, Si and Fe species in red mud during synthesis of one-part geopolymers. Cem Concr Res 101:123–130. https://doi.org/10.1016/j.cemconres.2017.08.027
Mukiza E, Liu X, Zhang L, Zhang Na (2019) Preparation and characterization of a red mud-based road base material: strength formation mechanism and leaching characteristics. Constr Build Mater 220:297–307. https://doi.org/10.1016/j.conbuildmat.2019.06.027
Shankar A, Kongot M, Saini VK, Kumar A (2020) Removal of pentachlorophenol pesticide from aqueous solutions using modified chitosan. Arab J Chem 13:1821–1830. https://doi.org/10.1016/j.arabjc.2018.01.016
Zhua Z, Yang Y, Fan Y, Zhang L, Tang S, Zhu Y, Zhou X (2022) Strontium-doped hydroxyapatite as an efficient adsorbent for Cd(II) removal from wastewater: performance, kinetics, and mechanism. Environ Technol Inno 28:102575. https://doi.org/10.1016/j.eti.2022.102575
Luu T-T, Dinh V-P, Nguyen Q-H, Tran N-Q, Nguyen D-K, Ho T-H, Nguyen V-D, Tran DX, Tuan Kieti HA (2022) Pb(II) adsorption mechanism and capability from aqueous solution using red mud modified by chitosan. Chemosphere 287(3):132279. https://doi.org/10.1016/j.chemosphere.2021.132279
Li Z, You H, Gao Y, Wang C, Zhang J (2021) Effect of ultrafine red mud on the workability and microstructure of blast furnace slag-red mud based geopolymeric grouts. Powder Technol 392:610–618. https://doi.org/10.1016/j.powtec.2021.07.046
Wang C, Li Z, Zhou Z, Gao Y, Zhang J (2022) Compatibility of different fibres with red mud-based geopolymer grouts. Constr Build Mater 315:125742. https://doi.org/10.1016/j.conbuildmat.2021.125742
Yan S, Ren X, Zhang F, Huang K, Feng X, Xing P (2022) Comparative study of Pb2+, Ni2+, and methylene blue adsorption on spherical waste solid-based geopolymer adsorbents enhanced with carbon nanotubes. Sep Purif Technol 284:120234. https://doi.org/10.1016/j.seppur.2021.120234
Nibou D, Mekatel H, Amokrane S, Barkat M, Trari M (2010) Adsorption of Zn2+ ions onto NaA and NaX zeolites: kinetic, equilibrium and thermodynamic studies. J Hazard Mater 173(1–3):637–646. https://doi.org/10.1016/j.jhazmat.2009.08.132
Novoselova LYu (2016) Hematite nanopowder obtained from waste: iron-removal sludge. Powder Technol 287:364–372. https://doi.org/10.1016/j.powtec.2015.10.020
Ye N, Yang J, Liang S, Yong Hu, Jingping Hu, Xiao Bo, Huang Q (2016) Synthesis and strength optimization of one-part geopolymer based on red mud. Constr Build Mater 111:317–325. https://doi.org/10.1016/j.conbuildmat.2016.02.099
Pepper RA, Couperthwaite SJ, Millar GJ (2016) Comprehensive examination of acid leaching behaviour of mineral phases from red mud: recovery of Fe, Al, Ti, and Si. Miner Eng 99:8–18. https://doi.org/10.1016/j.mineng.2016.09.012
Cui Y, Chen J, Zhang Y, Peng D, Huang T, Sun C (2019) pH-Dependent leaching characteristics of major and toxic elements from red mud. Int J Environ Res Public Health 16(11):2046. https://doi.org/10.3390/ijerph16112046
Zhang W, Liu X, Wang Y, Li Z, Li Y, Ren Y (2021) Binary reaction behaviors of red mud based cementitious material: hydration characteristics and Na+ utilization. J Hazard Mater 410:124592. https://doi.org/10.1016/j.jhazmat.2020.124592
Wang Li, Li J, Jin Y, Chen M, Luo J, Zhu X, Zhang Y (2019) Study on the removal of chromium(III) from leather waste by a two-step method. J Ind Eng Chem 79:172–180. https://doi.org/10.1016/j.jiec.2019.06.030
Zhang W, Feng Y, Niu Y, Liu T, Zhai X, Liu J (2021) Surface modification of superfine SiC powders by ternary modifiers-KH560/sodium humate/SDS and its mechanism. Ceram Internat 47(17):23834–23843. https://doi.org/10.1016/j.ceramint.2021.05.091
Zinchenko A, Sakai T, Morikawa K, Nakano M (2022) Efficient stabilization of soil, sand, and clay by a polymer network of biomass-derived chitosan and carboxymethyl cellulose. J Environ Chem Eng 10(1):107084. https://doi.org/10.1016/j.jece.2021.107084
Duncan Nowicki A, Janet Skakle MS, Iain Gibson R (2021) Maximising carbonate content in sodium-carbonate Co-substituted hydroxyapatites prepared by aqueous precipitation reaction. J Solid State Chem 297:122042. https://doi.org/10.1016/j.jssc.2021.122042
Shi W, Kataoka T, Hashimoto T, Liu Z, Tagaya M (2021) Competitive incorporation of Eu(III) and Na(I) ions into citric acid-passivated hydroxyapatite particles. Mater Lett 304:130561. https://doi.org/10.1016/j.matlet.2021.130561
Ma Ke, Cui H, Zhou A, Honghua Wu, Dong X, Fenghua Zu, Yi J, Wang R, Qinghong Xu (2021) Mesoporous hydroxyapatite: synthesis in molecular self-assembly and adsorption properties. Micropor Mesopor Mat 323:111164. https://doi.org/10.1016/j.micromeso.2021.111164
Davidovits J (2011) Geopolymer chemistry and applications, 3rd edn. Geopolymer Institute, Saint-Quentin
Ye N (2017) Study on the preparation of geopolymer from pretreated bay red mud and mechanism of the strength formation [D]. Huazhong university of science and technology, Wuhan
Garcia-Lodeiro I, Palomo A, Fernández-Jiménez A, Macphe DE (2011) Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem Concr Res 41:923–931. https://doi.org/10.1016/j.cemconres.2011.05.006
Li Z, Zhang J, Li S, Lin C, Gao Y, Liu C (2021) Feasibility of preparing red mud-based cementitious materials: synergistic utilization of industrial solid waste, waste heat, and tail gas. J Cleaner Prod 285:124896. https://doi.org/10.1016/j.jclepro.2020.124896
Zhang Na, Li H, Zhao Y, Liu X (2016) Hydration characteristics and environmental friendly performance of a cementitious material composed of calcium silicate slag. J Hazard Mater 306:67–76. https://doi.org/10.1016/j.jhazmat.2015.11.055
Puertas F, Palacios M, Manzano H, Dolado JS, Rico A, Rodríguez J (2011) A model for the C-A-S-H gel formed in alkali-activated slag cements. J Eur Ceram Soc 31(12):2043–2056. https://doi.org/10.1016/j.jeurceramsoc.2011.04.036
Qin Y, Chen X, Li B, Guo Y, Niu Z, Xia T, Meng W, Zhou M (2021) Study on the mechanical properties and microstructure of chitosan reinforced metakaolin-based geopolymer. Constr Build Mater 271:121522. https://doi.org/10.1016/j.conbuildmat.2020.121522
Acknowledgements
Authors would like to acknowledge the Major Scientific and Technological Innovation Projects in Shandong Province (Grants Nos. 2020CXGC011405 and Grants Nos. 2021CXGC010301), the National Key R&D Program of China (Grants Nos. 2022YFB2601900), and the Key Projects of Natural Science Foundation of Shandong Province (No. 2020KE006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no financial or non-financial interests to disclose.
Ethical approval
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., Li, Z., Zhang, C. et al. Study on occurrence form and solidification mechanism of alkaline components in red mud. J Mater Cycles Waste Manag 25, 3758–3775 (2023). https://doi.org/10.1007/s10163-023-01801-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01801-w