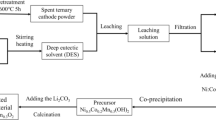
Abstract
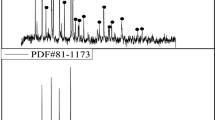
In this study, deep eutectic solvent synthesized by choline chloride (C5H14CINO) and oxalic acid dihydrate (C2H2O4·2H2O) was used to recycling LiFePO4 cathodes for spent lithium-ion batteries. The recycling process was optimized by response surface methodology. When the solid-to-liquid ratio is 0.02 and the reaction was performed at 106 °C for 110 min, the leaching efficiencies of lithium and iron are 95.3% and 85.2%, respectively. The measurement results show that iron is recycled as the form of FeC2O4·2H2O after the UV irradiation and lithium is recycled by chemical precipitation. No mineral acid or high temperature is involved in this work, which has great environmental significance to promote the healthy development of the battery recycling technology.
Graphical abstract






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sattar R, Ilyas S, Bhatti HN, Ghaffar A (2019) Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep Purif Technol 209:725–733
Xin C, Gao J, Luo R, Zhou W (2022) Prelithiation reagents and strategies on high energy lithium-ion batteries. Chemistry Eur J 28(23):e202104282
Nayak PK, Yang L, Brehm W, Adelhelm P (2018) From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises. Angew Chem Int Ed 57(1):102–120
Yu W, Guo Y, Shang Z, Zhang Y, Xu S (2022) A review on comprehensive recycling of spent power lithium-ion battery in China. Etransportation 11:100155
Harper G, Sommerville R, Kendrick E, Driscoll L, Slater P, Stolkin R, Walton A, Christensen P, Heidrich O, Lambert S, Abbott A, Ryder K, Gaines L, Anderson P (2019) Recycling lithium-ion batteries from electric vehicles. Nature 575(7781):75–86
Natarajan S, Aravindan V (2018) Burgeoning prospects of spent lithium-ion batteries in multifarious applications. Adv Energy Mater 8(33):1802303
Zhang J, Lei Y, Lin Z, Xie P, Lu H, Xu J (2022) A novel approach to recovery of lithium element and production of holey graphene based on the lithiated graphite of spent lithium ion batteries. Chem Eng J 436:135011
Lei Y, Zhang J, Chen X, Min W, Wang R, Yan M, Xu J (2022) From spent lithium-ion batteries to high performance sodium-ion batteries: a case study. Mater Today Energy 26:100997
Liu K, Tan Q, Liu L, Li J (2019) Acid-free and selective extraction of lithium from spent lithium iron phosphate batteries via a mechanochemically induced isomorphic substitution. Environ Sci Technol 53(16):9781–9788
Medić D, Sokić M, Nujkić M, Đorđievski S, Milić S, Alagić S, Antonijević M (2023) Cobalt extraction from spent lithium-ion battery cathode material using a sulfuric acid solution containing SO2. J Mater Cycles Waste Manag 25:1008–1018
Sasai R, Fujimura T, Uesugi R, Aketo T, Komatsu K (2023) Eco-friendly recovery process of lithium from reduction roasting residue powder based on hydrothermal extraction. J Mater Cycles Waste Manage 25(1):389–395
Wang WY, Yen CH, Lin JL, Xu RB (2019) Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J Mater Cycles Waste Manage 21(2):300–307
Zhang P, Yokoyama T, Itabashi O, Wakui Y, Inoue K (1998) Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 50(1):61–75
Chen L, Tang X, Zhang Y, Li L, Zeng Z, Zhang Y (2011) Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 108(1–2):80–86
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sources 195(4):939–954
Fan E, Li L, Zhang X, Bian Y, Xue Q, Wu J, Wu F, Chen R (2018) Selective recovery of Li and Fe from spent lithium-ion batteries by an environmentally friendly mechanochemical approach. ACS Sustain Chem Eng 6(8):11029–11035
Chen JP, Li QW, Song JS, Song DW, Zhang LQ, Shi XX (2016) Environmentally friendly recycling and effective repairing of cathode powders from spent LiFePO4 batteries. Green Chem 18(8):2500–2506
Qiu XJ, Zhang BC, Xu YL, Hu JG, Deng WT, Zou GQ, Hou HS, Yang Y, Sun W, Hu YH, Cao XY, Ji XB (2022) Enabling the sustainable recycling of LiFePO4 from spent lithium-ion batteries. Green Chem 24(6):2506–2515
Liu P, Fei Z, Zhang Y, Dong P, Meng Q, Yang X (2022) Efficient oxidation approach for selective recovery of lithium from cathode materials of spent LiFePO4 batteries. Jom 74(5):1934–1944
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114(21):11060–11082
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126(29):9142–9147
Abbott AP, Capper G, Davies DL, McKenzie KJ, Obi SU (2006) Solubility of metal oxides in deep eutectic solvents based on choline chloride. J Chem Eng Data 51:1280–1282
Zürner P, Frisch G (2019) Leaching and selective extraction of indium and tin from zinc flue dust using an oxalic acid-based deep eutectic solvent. ACS Sustain Chem Eng 7(5):5300–5308
Tang S, Zhang M, Guo M (2022) A novel deep-eutectic solvent with strong coordination ability and low viscosity for efficient extraction of valuable metals from spent lithium-ion batteries. ACS Sustain Chem Eng 10:975–985
Wang H, Li M, Garg S, Wu Y, Nazmi Idros M, Hocking R, Duan H, Gao S, Yago AJ, Zhuang L, Rufford TE (2021) Cobalt electrochemical recovery from lithium cobalt oxides in deep eutectic choline chloride+urea solvents. Chemsuschem 14(14):2972–2983
Chen Y, Lu Y, Liu Z, Zhou L, Li Z, Jiang J, Wei L, Ren P, Mu T (2020) Efficient dissolution of lithium-ion batteries cathode LiCoO2 by polyethylene glycol-based deep eutectic solvents at mild temperature. ACS Sustain Chem Eng 8(31):11713–11720
Tran MK, Rodrigues M-TF, Kato K, Babu G, Ajayan PM (2019) Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat Energy 4:339–345
Jin H, Zhang J, Wang D, Jing Q, Chen Y, Wang C (2022) Facile and efficient recovery of lithium from spent LiFePO4 batteries via air oxidation–water leaching at room temperature. Green Chem 24(1):152–162
Liu X, Li T, Wu S, Ma H, Yin Y (2020) Structural characterization and comparison of enzymatic and deep eutectic solvents isolated lignin from various green processes: toward lignin valorization. Biores Technol 310:123460
Jha MK, Kumari A, Jha AK, Kumar V, Hait J, Pandey BD (2013) Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manage 33(9):1890–1897
Wang M, Tan Q, Liu L, Li J (2019) A low-toxicity and high-efficiency deep eutectic solvent for the separation of aluminum foil and cathode materials from spent lithium-ion batteries. J Hazard Mater 380:120846
Heydarian A, Mousavi SM, Vakilchap F, Baniasadi M (2018) Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. J Power Sources 378:19–30
Pozdnyakov IP, Kel OV, Plyusnin VF, Grivin VP, Bazhin NM (2008) New insight into photochemistry of ferrioxalate. J Phys Chem A 112(36):8316–8322
Taxiarchou M, Panias D, Douni I, Paspaliaris I, Kontopoulos A (1997) Removal of iron from silica sand by leaching with oxalic acid. Hydrometallurgy 46(1):215–228
Taxiarchou M, Panias D, Douni I, Paspaliaris I, Kontopoulos A (1997) Dissolution of hematite in acidic oxalate solutions. Hydrometallurgy 44(3):287–299
Nazrul Islam SMK, Asw K, Gulshan F (2013) Photocatalytic efficiency of mill scale for the degradation of textile dye by photo fenton and photo-ferrioxalate system under UV and sunlight. Environ Ecol Res 1:129–134
Zheng R, Zhao L, Wang W, Liu Y, Ma Q, Mu D, Li R, Dai C (2016) Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Adv 6(49):43613–43625
Yu Z, Shi Z, Chen Y, Niu Y, Wang Y, Wan P (2012) Red-mud treatment using oxalic acid by UV irradiation assistance. Trans Nonferrous Metals Soc China 22(2):456–460
Lahiri A (2010) Influence of ascorbate and oxalic acid for the removal of iron and alkali from alkali roasted ilmenite to produce synthetic rutile. Ind Eng Chem Res 49(18):8847–8851
Chen J, Zhang H, Tomov IV, Wolfsberg M, Ding X, Rentzepis PM (2007) Transient structures and kinetics of the ferrioxalate redox reaction studied by time-resolved EXAFS, optical spectroscopy, and DFT. J Phys Chem A 111(38):9326–9335
Acknowledgements
The work was supported by the Central Guidance on Local Science and Technology Development Fund of Henan Province (Z20221343028), Independent Innovation Fund Project of Wuhan University of Technology (2019III123CG), 111 Project (B17034) and Innovative Research Team Development Program of Ministry of Education of China (IRT_17R83). XRD, UV-VIS and FT-IR examinations were assisted by the Center of Material Research and Analysis of Wuhan University of Technology. Thanks to Wuhan GEM Co., Ltd., China for its strong support.
Author information
Authors and Affiliations
Contributions
CW: Methodology, conceptualization, writing—review and editing, supervision, project administration. HY: Formal analysis, investigation, data curation, writing—original draft. CY: Validation, supervision, project administration, funding acquisition. YL: Conceptualization, methodology, resources, investigation, writing- review and editing, supervision, project administration, funding acquisition. LB: Project administration, funding acquisition. SY: Supervision, project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Yang, H., Yang, C. et al. A novel recycling process of LiFePO4 cathodes for spent lithium-ion batteries by deep eutectic solvents. J Mater Cycles Waste Manag 25, 2077–2086 (2023). https://doi.org/10.1007/s10163-023-01654-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01654-3