Abstract
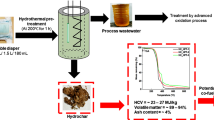
Portlandite (Ca(OH)2) preparation from phosphogypsum waste (PGW) was evaluated in numerous works; however, the use of this compound is not applied yet in the brine water salinity reduction. In this work, the purity of Ca(OH)2 prepared from PGW and soda aqueous solution was tested for different NaOH/PG molar ratios (MR) close to the stochiometric value (1.5 < MR < 3). The obtained Ca(OH)2 at the optimal conditions was used for both the CO2 capture and brine water (BW) salinity reduction, resulting in high-quality calcium carbonate synthesizing. The XRD results show that a MR higher than the stochiometric value (MR > 2) is most efficient to obtain high-quality portlandite (P2.5 and P3). An estimation of the carbon capture efficiency of the prepared portlandite was made. Based on the results of this work, using 4 g of P2.5 allows to capture 2.4 L of CO2 and treat 0.4 L of BW. Overall, our work confirms the technical viability of the proposed route to synergize CO2 capture, BW and PGW recycling. From an economic and ecological standpoint, our investigated routes were eco-friendly and cost-effective.







Similar content being viewed by others
Availability of data and materials
All data derived during the experiments are given in the paper and referenced.
Abbreviations
- BW:
-
Brine water
- EC:
-
Electrical conductivity
- MR:
-
Molar ratio
- P2.5 :
-
Portlandite prepared using MR = 2.5
- P3 :
-
Portlandite prepared using MR = 3
- PGW:
-
Phosphogypsum waste
- XRD:
-
X-ray diffraction
References
Bouargane B, Marrouche A, El IS et al (2019) Recovery of Ca(OH)2, CaCO3, and Na2SO4 from Moroccan phosphogypsum waste. J Mater Cycles Waste Manag. https://doi.org/10.1007/s10163-019-00910-9
Abu-Eishah SI, Bani-Kananeh AA, Allawzi MA (2000) K2SO4 production via the double decomposition reaction of KCl and phosphogypsum. Chem Eng J 76:197–207. https://doi.org/10.1016/S1385-8947(99)00158-8
Abbas KK (2011) Study on the production of ammonium sulfate fertlizer from phosphogypsum. Eng Tech J. 29:814–821
Idboufrade A, Bouargane B, Ennasraoui B et al (2021) Phosphogypsum two-step ammonia-carbonation resulting in ammonium sulfate and calcium carbonate synthesis: effect of the molar ratio OH−/Ca2+ on the conversion process. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-021-01600-0
Romero-Hermida I, Santos A, Pérez-López R et al (2017) New method for carbon dioxide mineralization based on phosphogypsum and aluminium-rich industrial wastes resulting in valuable carbonated by-products. J CO2 Util 18:15–22. https://doi.org/10.1016/j.jcou.2017.01.002
El Housse M, Abdallah H, Ilham K et al (2021) Study of the effect of inorganic inhibitor on the calcium carbonate precipitation in the localized irrigation systems. Nanotechnol Environ Eng 2:1–9. https://doi.org/10.1007/s41204-021-00107-2
Karmal I, Mohareb S, El Housse M et al (2020) Structural and morphological characterization of scale deposits on the reverse osmosis membranes: case of brackish water demineralization station in Morocco. Groundw Sustain Dev 11:100483. https://doi.org/10.1016/j.gsd.2020.100483
Bouargane B, Biyoune MG, Mabrouk A et al (2020) Experimental investigation of the effects of synthesis parameters on the precipitation of calcium carbonate and portlandite from Moroccan phosphogypsum and pure gypsum using carbonation route. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-019-00923-3
Douahem H, Hammi H, Hamzaoui AH, Nif AM (2017) A preliminary study of phosphogypsum transformation into calcium fluoride. J Tunis Chem Soc 19:147–151
Smol M (2019) The importance of sustainable phosphorus management in the circular economy (CE) model: the Polish case study. J Mater Cycles Waste Manag 21:227–238. https://doi.org/10.1007/s10163-018-0794-6
Abril JM, García-Tenorio R, Enamorado SM et al (2008) The cumulative effect of three decades of phosphogypsum amendments in reclaimed marsh soils from SW Spain: 226Ra, 238U and Cd contents in soils and tomato fruit. Sci Total Environ 403:80–88. https://doi.org/10.1016/j.scitotenv.2008.05.013
Alcordo IS, Rechcigl JE (1993) Phosphogypsum in agriculture: a review. Adv Agron 49:55–118. https://doi.org/10.1016/S0065-2113(08)60793-2
Kacimi L, Simon-Masseron A, Ghomari A, Derriche Z (2006) Reduction of clinkerization temperature by using phosphogypsum. J Hazard Mater 137:129–137. https://doi.org/10.1016/j.jhazmat.2005.12.053
Liu DS, Wang CQ, Mei XD, Zhang C (2019) An effective treatment method for phosphogypsum. Environ Sci Pollut Res 26:30533–30539. https://doi.org/10.1007/s11356-019-06113-x
Shen W, Gan G, Dong R et al (2012) Utilization of solidified phosphogypsum as Portland cement retarder. J Mater Cycles Waste Manag 14:228–233. https://doi.org/10.1007/s10163-012-0065-x
Elfadil S, Hamamouch N, Jaouad A et al (2020) The effect of phosphate flotation wastes and phosphogypsum on cattle manure compost quality and plant growth. J Mater Cycles Waste Manag 22:996–1005. https://doi.org/10.1007/s10163-020-00997-5
Mymrin V, Aibuldinov EK, Avanci MA et al (2021) Material cycle realization by hazardous phosphogypsum waste, ferrous slag, and lime production waste application to produce sustainable construction materials. J Mater Cycles Waste Manag 23:591–603. https://doi.org/10.1007/s10163-020-01147-7
Contreras M, Pérez-López R, Gázquez MJ et al (2014) Fractionation and fluxes of metals and radionuclides during the recycling process of phosphogypsum wastes applied to mineral CO2 sequestration. Waste Manag 45:412–419. https://doi.org/10.1016/j.wasman.2015.06.046
Cárdenas-Escudero C, Morales-Flórez V, Pérez-López R et al (2011) Procedure to use phosphogypsum industrial waste for mineral CO2 sequestration. J Hazard Mater 196:431–435. https://doi.org/10.1016/j.jhazmat.2011.09.039
Rosales J, Pérez SM, Cabrera M et al (2020) Treated phosphogypsum as an alternative set regulator and mineral addition in cement production. J Clean Prod 244:118752. https://doi.org/10.1016/j.jclepro.2019.118752
Amrani M, Taha Y, Kchikach A et al (2020) Phosphogypsum recycling: new horizons for a more sustainable road material application. J Build Eng. 30:101267. https://doi.org/10.1016/j.jobe.2020.101267
Chen Q, Zhang Q, Qi C et al (2018) Recycling phosphogypsum and construction demolition waste for cemented paste backfill and its environmental impact. J Clean Prod 186:418–429. https://doi.org/10.1016/j.jclepro.2018.03.131
Pereira VM, Geraldo RH, Cruz TAM, Camarini G (2021) Valorization of industrial by-product: phosphogypsum recycling as green binding material. Clean Eng Technol. 5:100310. https://doi.org/10.1016/j.clet.2021.100310
Wu F, Ren Y, Qu G et al (2022) Utilization path of bulk industrial solid waste: a review on the multi-directional resource utilization path of phosphogypsum. J Environ Manage 313:114957. https://doi.org/10.1016/j.jenvman.2022.114957
El-Naas MH, Al-Marzouqi AH, Chaalal O (2010) A combined approach for the management of desalination reject brine and capture of CO2. Desalination 251:70–74. https://doi.org/10.1016/j.desal.2009.09.141
Danoun R (2007) Desalination Plants: Potential impacts of brine discharge on marine life. Final Proj Ocean Technol Gr 59. https://ses.library.usyd.edu.au/handle/2123/1897
El-Naas MH, Mohammad AF, Suleiman MI et al (2017) A new process for the capture of CO2 and reduction of water salinity. Desalination 411:69–75. https://doi.org/10.1016/j.desal.2017.02.005
Dindi A, Quang DV, AlNashef I, Abu-Zahra MRM (2018) A process for combined CO2 utilization and treatment of desalination reject brine. Desalination 442:62–74. https://doi.org/10.1016/j.desal.2018.05.014
He K, Tang Z, Song Q, Yao Q (2022) Process analysis of SO3 removal by Ca(OH)2 particles from flue gas. Chem Eng Sci 247:117054. https://doi.org/10.1016/j.ces.2021.117054
Kwak Y-H, Lee Y-M, Bae W-K et al (2011) Absorption behavior of chlorine and sulfur by Ca(OH)2 under simulated conditions with a fluidized bed combustor. J Mater Cycles Waste Manag 13:314–320. https://doi.org/10.1007/s10163-011-0030-0
Shemer H, Hasson D, Semiat R et al (2013) Remineralization of desalinated water by limestone dissolution with carbon dioxide. Desalin Water Treat 51:877–881. https://doi.org/10.1080/19443994.2012.694236
Vats G, Mathur R (2022) A net-zero emissions energy system in India by 2050: an exploration. J Clean Prod 352:131417. https://doi.org/10.1016/j.jclepro.2022.131417
Biyoune MG, Bouargane B, Idboufrade A et al (2021) New procedure for water-salinity reduction using Phosphogypsum waste and carbon dioxide resulting in useful compounds formation. Nanotechnol Environ Eng 6:1–18. https://doi.org/10.1007/s41204-021-00125-0
Marrouche A, Bouargane B, Mabrouk A et al (2019) Solubility in the ternary system MgCl2-FeCl2-H2O at 288 K by conductance method. Mediterr J Chem. 8:10. https://doi.org/10.13171/mjc811902523am
Chen L, Msigwa G, Yang M et al (2022) Strategies to achieve a carbon neutral society: a review. Environ Chem Lett 20:2277–2310. https://doi.org/10.1007/s10311-022-01435-8
Ho H-J, Iizuka A, Lee C-H, Chen W-S (2022) Mineral carbonation using alkaline waste and byproducts to reduce CO2 emissions in Taiwan. Environ Chem Lett. https://doi.org/10.1007/s10311-022-01518-6
Woodall CM, Mcqueen N, Pilorgé H, Wilcox J (2019) Utilization of mineral carbonation products: current state and potential. Greenh Gas Sci Technol 9:1096–1113. https://doi.org/10.1002/ghg.1940
Avşar C, Tümük D, Yüzbaşıoğlu AE, Gezerman AO (2022) Focusing on the Merseburg process: benefits on industrial decarbonization and waste minimization. Environ Technol Rev 11:148–155. https://doi.org/10.1080/21622515.2022.2119171
Avşar C, Gezerman AO (2022) Industrial waste management : economical benefits of the resource utilization of phosphogypsum. Int J Ind Mark 7:1–9. https://doi.org/10.5296/ijim.v7i1.20325
Liu W, Teng L, Rohani S et al (2021) CO2 mineral carbonation using industrial solid wastes: a review of recent developments. Chem Eng J 416:129093. https://doi.org/10.1016/j.cej.2021.129093Received
U.S. Geological Survey (2020) Mineral commodity summaries 2020: U.S. Geological Survey. 200 https://doi.org/10.3133/mcs2020
Zdah I, Azifa A, Ennaciri Y et al (2022) Green method of phosphogypsum waste conversion to lithium sulfate monohydrate and calcium hydroxide. Sustain Chem Pharm. 30:100850. https://doi.org/10.1016/j.scp.2022.100850
Altiner M (2018) Effect of alkaline types on the production of calcium carbonate particles from gypsum waste for fixation of CO2 by mineral carbonation. Int J Coal Prep Util 39:1–19. https://doi.org/10.1080/19392699.2018.1452739
Acknowledgements
The authors would like to acknowledge the support provided by the University of Ibn Zohr, Faculty of sciences Agadir through the research project APUiz/2018.
Author information
Authors and Affiliations
Contributions
BB: investigation, experimental methodology and visualization, and writing original draft, MGB: investigation, methodology, conceptualization, visualization, and writing original draft. MSP: characterization and analysis. BB: writing—review and editing. AA: visualization, review and editing, supervision, project administration, resources. JPBR: supervision, project administration, review and editing, and resources.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethics approval
Not applicable.
Consent to participate
The authors consent to participate in the publication of this manuscript at the Journal of Material Cycles and Waste Management (JMCWM).
Consent to publish
All authors have checked the manuscript and have agreed to the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouargane, B., Biyoune, M.G., Pérez Moreno, S. et al. Portlandite wet-synthesis process from phosphogypsum waste using hydroxide medium: application in both CO2 capture and brine water salinity reduction. J Mater Cycles Waste Manag 25, 1771–1780 (2023). https://doi.org/10.1007/s10163-023-01590-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01590-2