Abstract
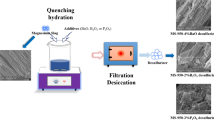
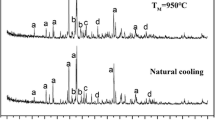
The original magnesium slag has poor desulfurization activity, so modification treatments are needed to improve its desulfurization activity. In this article, magnesium slag was modified by quenching hydration. The crystal structure and morphology characteristics were obtained by XRD and TEM. And to further understand the relationship between crystal structure and desulfurization performance, desulfurization experiments were carried out by TGA. A theoretical basis for future development of desulfurization methods was provided, which could not only alleviate the disposal problem but also help control SO2 pollution. The results showed that the naturally cooled magnesium slag was mainly composed of γ-C2S and β-C2S. The irregular coordination of Ca2+ in β-C2S was the main reason that the hydration activity of β-C2S was higher than that of γ-C2S. With the increase of quenching temperature, the β-C2S content increased continuously, promoting the formation of C–S–H with developed pore structure. With the increasing hydration temperature, the hydration reaction of β-C2S could be promoted, and more amorphous C–S–H could be generated. The hydration product C–S–H enhanced the physical adsorption capacity, and the increase of crystal structure defects enhanced the chemical adsorption capacity. At 950 °C quenching temperature and 90 °C hydration temperature, the desulfurization activity was the highest.
Graphic abstract















Similar content being viewed by others
References
de Correia NS, Caldas RCS, Oluremi JR (2020) Feasibility of using CDW fine fraction and bentonite mixtures as alternative landfill barrier material. J Mater Cycles Waste Manag 22:1877–1886. https://doi.org/10.1007/s10163-020-01075-6
Song ZJ, Zeng JH, Wang C, Zhang J, Zhou JH, Pan Y, Xu YF, Liu Q, Qian GR (2020) Application and mechanism of an ore-washing sludge in the remediation of chromium (III) and copper (II)-contaminated soils. J Mater Cycles Waste Manag 22:897–906. https://doi.org/10.1007/s10163-020-00980-0
Pantazopoulou E, Ntinoudi E, Zouboulis AI, Mitrakas M, Yiannoulakis H, Zampetakis T (2020) Heavy metal stabilization of industrial solid wastes using low-grade magnesia, Portland and magnesia cements. J Mater Cycles Waste Manag 22:975–985. https://doi.org/10.1007/s10163-020-00985-9
Nguyen H, Moghadam MJ, Moayedi H (2019) Agricultural wastes preparation, management, and applications in civil engineering: a review. J Mater Cycles Waste Manag 21:1039–1051. https://doi.org/10.1007/s10163-019-00872-y
Rahman MT, Kameda T, Miura T, Kumagai S, Yoshioka T (2019) Removal of Mn and Cd contained in mine wastewater by Mg-Al-layered double hydroxides. J Mater Cycles Waste Manag 21:1232–1241. https://doi.org/10.1007/s10163-019-00875-9
Lee S, Lee M-G, Park J (2018) Catalytic upgrading pyrolysis of pine sawdust for bio-oil with metal oxides. J Mater Cycles Waste Manag 20:1553–1561. https://doi.org/10.1007/s10163-018-0716-7
Li HX, Huang YY, Yang XJ, Jiang ZW, Yang ZH (2018) Approach to the management of magnesium slag via the production of Portland cement clinker. J Mater Cycles Waste Manag 20:1701–1709. https://doi.org/10.1007/s10163-018-0735-4
Sun Y, Meng Y, Guo XY, Zhu TL, Liu HJ, Li WP (2016) Study of nitrogen oxide absorption in the calcium sulfite slurry. J Mater Cycles Waste Manag 18:618–624. https://doi.org/10.1007/s10163-016-0526-8
Mao LQ, Cui H, Miao CC, An H, Zhai JP, Li Q (2016) Preparation of MgCr2O4 from waste tannery solution and effect of sulfate, chloride, and calcium on leachability of chromium. J Mater Cycles Waste Manag 18:573–581. https://doi.org/10.1007/s10163-015-0354-2
Ren Y, Kang S, Zhu J (2015) Mechanochemical degradation of hexachlorobenzene using Mg/Al2O3 as additive. J Mater Cycles Waste Manag 17:607–615. https://doi.org/10.1007/s10163-015-0398-3
Cabrera-Real H, Romero-Serrano A, Zeifert B, Hernandez-Ramirez A, Hallen-Lopez M, Cruz-Ramirez A (2012) Effect of MgO and CaO/SiO2 on the immobilization of chromium in synthetic slags. J Mater Cycles Waste Manag 14:317–324. https://doi.org/10.1007/s10163-012-0072-y
Hong MH, Joo SY, Kim S, Lee CG, Kim DW, Yoon JH (2020) Asbestos-containing waste detoxification by a microwave heat treatment using silicon carbide as an inorganic heating element. J Mater Cycles Waste Manag 22:826–835. https://doi.org/10.1007/s10163-020-00977-9
Ha S, Lee JW, Choi SH, Kim SH, Kim K, Kim Y (2019) Calcination characteristics of oyster shells and their comparison with limestone from the perspective of waste recycling. J Mater Cycles Waste Manag 21:1075–1084. https://doi.org/10.1007/s10163-019-00860-2
Back SK, Mojammal AHM, Jo HH, Kim JH, Jeong MJ, Seo YC, Joung HT, Kim SH (2019) Increasing seawater alkalinity using fly ash to restore the pH and the effect of temperature on seawater flue gas desulfurization. J Mater Cycles Waste Manag 21:962–973. https://doi.org/10.1007/s10163-019-00852-2
Fan BG, Jia L, Li B, Huo RP, Yao YX, Han F, Qiao XL, Jin Y (2018) Study on desulfurization performances of magnesium slag with different hydration modification. J Mater Cycles Waste Manag 20:1771–1780. https://doi.org/10.1007/s10163-018-0744-3
Fan BG, Jia L, Han F, Huo RP, Yao YX, Qiao XL, Zhao CW, Jin Y (2019) Study on magnesium slag desulfurizer modified by additives in quenching hydration. J Mater Cycles Waste Manag 21:1211–1223. https://doi.org/10.1007/s10163-019-00877-7
Jia L, Fan BG, Huo RP, Li B, Yao YX, Han F, Qiao XL, Jin Y (2018) Study on quenching hydration reaction kinetics and desulfurization characteristics of magnesium slag. J Clean Prod 190:12–23. https://doi.org/10.1016/j.jclepro.2018.04.150
Fan BG, Jin Y, Zheng XR, Qiao XL, Wang XT (2012) Experimental study on the desulfurization performance of magnesium slag. In: Qi H, Zhao B (eds) Cleaner combustion and sustainable world. Springer-verlag Berlin, Germany, pp 344–347
Dietel J, Groeger-Trampe J, Bertmer M, Kaufhold S, Ufer K, Dohrmann R (2019) Crystal structure model development for soil clay minerals - I. Hydroxy-interlayered smectite (HIS) synthesized from bentonite. A multi-analytical study. Geoderma 347:135–149. https://doi.org/10.1016/j.geoderma.2019.03.021
Jia L, Fan BG, Yao YX, Han F, Huo RP, Zhao CW, Jin Y (2018) Study on the elemental mercury adsorption characteristics and mechanism of iron-based modified biochar materials. Energy Fuels 32:12554–12566. https://doi.org/10.1021/acs.energyfuels.8b02890
Wada Y, Fujii S, Suzuki E, Maitani MM, Tsubaki S, Chonan S, Fukui M, Inazu N (2017) Smelting magnesium metal using a microwave pidgeon method. Sci Rep. https://doi.org/10.1038/srep46512
Gregurek D, Peng Z, Wenzl C, White JF (2016) Metal smelting and furnace tapping. JOM 68:1516–1517. https://doi.org/10.1007/s11837-016-1925-y
Honda H (2007) Ionic dynamics in the ionic plastic crystal NH4NO2. Zeitschrift Fur Naturforsch Sect A-A J Phys Sci 62:633–638. https://doi.org/10.1515/zna-2007-10-1112
Bugaenko LT, Zakharov YA, Ryabykh SM, Yakubik DG (2007) Radiation resistance of ionic and ionic-molecular crystals. High Energy Chem 41:324–332. https://doi.org/10.1134/S0018143907050049
Wang QQ, Li F, Shen XD, Shi WJ, Li XR, Guo YH, Xiong SJ, Zhu Q (2014) Relation between reactivity and electronic structure for alpha `(L-), beta- and gamma-dicalcium silicate: a first-principles study. Cem Concr Res 57:28–32. https://doi.org/10.1016/j.cemconres.2013.12.004
Kriskova L, Pontikes Y, Zhang F, Cizer O, Jones PT, Van Balen K, Blanpain B (2014) Influence of mechanical and chemical activation on the hydraulic properties of gamma dicalcium silicate. Cem Concr Res 55:59–68. https://doi.org/10.1016/j.cemconres.2013.10.004
Schoff CK (2006) Surface tension and surface energy. Jct Coatings Tech 3:72
Frankcombe TJ, Collins MA (2011) Potential energy surfaces for gas-surface reactions. Phys Chem Chem Phys 13:8379–8391. https://doi.org/10.1039/c0cp01843k
Birol Y (2006) Grain refining efficiency of Al-Ti-C alloys. J Alloys Compd 422:128–131. https://doi.org/10.1016/j.jallcom.2005.11.059
Bermingham MJ, McDonald SD, St John DH, Dargusch MS (2010) Titanium as an endogenous grain-refining nucleus. Philos Mag 90:699–715. https://doi.org/10.1080/14786430903236057
Partch R (2007) Aerogel materials. Chem Eng News 85:10
Jia L, Fan BG, Zheng XR, Qiao XL, Yao YX, Zhao R, Guo JR, Jin Y (2021) Mercury emission and adsorption characteristics of fly ash in PC and CFB boilers. Front Energy 15:112–123. https://doi.org/10.1007/s11708-020-0682-3
Jia L, Fan BG, Li B, Yao YX, Huo RP, Zhao R, Qiao XL, Jin Y (2018) Effects of pyrolysis mode and particle size on the microscopic characteristics and mercury adsorption characteristics of biomass char. BioResources 13:5450–5471
Acknowledgements
The authors acknowledge the financial support for this work provided by National Natural Science Foundation of China (No. U1510135), National Natural Science Foundation of China (No. U1810126), National Natural Science Foundation of China (No. U1910214), Shanxi Province Science and Technology Innovation Project of Colleges and Universities (No. 2020L0073), Project (SKLD21KM16) supported by State Key Laboratory of Power System and Generation Equipment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jia, L., Han, F., Guo, Jr. et al. Crystal structure of a new high-performance magnesium slag desulfurizer modified by quenching hydration. J Mater Cycles Waste Manag 24, 210–223 (2022). https://doi.org/10.1007/s10163-021-01311-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-021-01311-7