Abstract
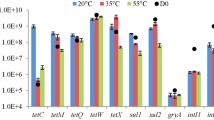
Anaerobic digestion is known to eliminate many kinds of microorganisms in livestock wastes. In this study, the quantitative changes of Pseudomonas species during mesophilic and thermophilic anaerobic digestions were investigated with focus on P. aeruginosa and P. fluorescens, which are known as a pathogen and a plant growth-promoting rhizobacterium, respectively. Furthermore, quantitative changes in antimicrobial-resistant Pseudomonas species against cefazolin were also investigated as a representative of antimicrobial resistance in dairy farms. The quantitative measurement of Pseudomonas species was performed by a plating method with modified Pseudomonas-selective agar medium. The colonies on the agar plates were classified into three groups by their colour development and constitutional fluorescence, and the group to which P. aeruginosa and P. fluorescens belong was confirmed by colony PCR using species-specific primers. The results revealed that while the number of total Pseudomonas decreased, the number of fluorescent Pseudomonas including P. fluorescens increased after anaerobic digestion. The abundance of cefazolin-resistant Pseudomonas also decreased, and no P. aeruginosa could be detected in any samples. These results suggested that the digestate has benefits such as the absence of pathogenic risks led by the Pseudomonas species and is expected to have a plant growth-promoting effect facilitated by fluorescent Pseudomonas.



Similar content being viewed by others
References
Sumathi BR, Veeregowda BM, Amitha RG (2008) Prevalence and antibiogram profile of bacterial isolates from clinical bovine mastitis. Vet World 1:237–238
Jackson CR, Lombard JE, Dargatz DA, Fedorka-Cray PJ (2011) Prevalence, species distribution and antimicrobial resistance of enterococci isolated from US dairy cattle. Lett Appl Microbiol 52:41–48. https://doi.org/10.1111/j.1472-765X.2010.02964.x
Ohnishi M, Sawada T, Hirose K, Sato R, Hayashimoto M, Hata E, Yonezawa C, Kato H (2011) Antimicrobial susceptibilities and bacteriological characteristics of bovine Pseudomonas aeruginosa and Serratia marcescens isolates from Mastitis. Vet Microbiol 154:202–207. https://doi.org/10.1016/j.vetmic.2011.06.023
Wagner S, Erskine R (2013) Antimicrobial drug use in mastitis. In: Giguère S, Prescott JF, Dowling PM (eds) Antimicrobial therapy in veterinary medicine, 5th edn. Wiley, Hoboken, pp 519–528
Misaghi I, Grogan RG (1969) Nutritional and biochemical comparisons of plant-pathogenic and saprophytic fluorescent pseudomonads. Phytopathology 59:1436–1450
Alippi AM, Dal Bo E, Ronco LB, López MV, López AC, Aguilar OM (2003) Aguilar, Pseudomonas populations causing pith necrosis of tomato and pepper in Argentina are highly diverse. Plant Pathol 52:287–302. https://doi.org/10.1046/j.1365-3059.2003.00850.x
Zaccardelli M, Spasiano A, Bazzi C, Merighi M (2005) Identification and in planta detection of Pseudomonas syringae pv. tomato using PCR amplification of hrpZPst. Eur J Plant Pathol 111:85–90. https://doi.org/10.1007/s10658-004-2734-7
Cawoy H, Bettiol W, Fickers P, Ongena M (2011) Bacillus-based biological control of plant diseases. Pesticides in the modern world—pesticides use and management. InTech, Rijeka, pp 273–302
De Brito AM, Gagne S, Antoun H (1995) Effect of compost on rhizosphere microflora of the tomato and on the incidence of plant growth-promoting rhizobacteria. Appl Environ Microbiol 61:194–199
Hameeda B, Rupela OP, Reddy G, Satyavani K (2006) Application of plant growth-promoting bacteria associated with composts and macrofauna for growth promotion of Pearl millet (Pennisetum glaucum L.). Biol Fertil Soils 43:221–227. https://doi.org/10.1007/s00374-006-0098-1
Mishra PK, Mishra S, Selvakumar G, Bisht JK, Kundu S, Gupta HS (2009) Coinoculation of Bacillus thuringeinsis-KR1 with Rhizobium leguminosarum enhances plant growth and nodulation of pea (Pisum sativum L.) and lentil (Lens culinaris L.). World J Microbiol Biotechnol 25:753–761. https://doi.org/10.1007/s11274-009-9963-z
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125. https://doi.org/10.1016/j.tim.2007.12.009
Brandl MT, Quinones B, Lindow SE (2001) Heterogeneous transcription of an indoleacetic acid biosynthetic gene in Erwinia herbicola on plant surfaces. Proc Natl Acad Sci USA 98:3454–3459. https://doi.org/10.1073/pnas.061014498
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319. https://doi.org/10.1038/nrmicro1129
Laville J, Voisard C, Keel C, Maurhofer M, Defago G, Haas D (1992) Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci USA 89:1562–1566. https://doi.org/10.1073/pnas.89.5.1562
Vidhyasekaran P, Muthamilan M (1995) Development of formulations of Pseudomonas fluorescens for control of chickpea wilt. Plant Dis 79:782–786
Botelho GR, Mendonça-Hagler LC (2006) Fluorescent Pseudomonads associated with the rhizosphere of crops—an overview. Braz J Microbiol 37:401–416. https://doi.org/10.1590/S1517-83822006000400001
Iwamoto Y, Aino M (2008) Suppressive effect of Pseudomonas fluorescens FPH9601 on crown and root rot disease of tomato caused by Fusarium oxysporum f.sp. radicis-lycopersici (in Japanese). Soil Microorg 62:3–8
Yamashiro T, Lateef SA, Ying C, Beneragama N, Lukic M, Iwasaki M, Ihara I, Nishida T, Umetsu K (2013) Anaerobic co-digestion of dairy cow manure and high concentrated food processing waste. J Mater Cycles Waste Manag 15:539–547. https://doi.org/10.1007/s10163-012-0110-9
Iwasaki M, Yamashiro T, Beneragama N, Nishida T, Kida K, Ihara I, Takahashi J, Umetsu K (2011) The effect of temperature on survival of pathogenic bacteria in biogas plant. Anim Sci J 82:707–712. https://doi.org/10.1111/j.1740-0929.2011.00887.x
Stanbridge LH, Board RG (1994) A modification of the Pseudomonas selective medium, CFC, that allows differentiation between meat pseudomonads and Enterobacteriaceae. Lett Appl Microbiol 18:327–328. https://doi.org/10.1111/j.1472-765X.1994.tb00880.x
Stalon V, Mercenier A (1984) l-Arginine utilization by Pseudomonas species. J Gen Microbiol 130:69–76. https://doi.org/10.1099/00221287-130-1-69
da Silva Filho LVF, Levi JE, Bento CNO, Ramos SRTS, Rozov T (1999) PCR identification of Pseudornonas aeruginosa and direct detection in clinical samples from cystic fibrosis patients. J Med Microbiol 48:357–361. https://doi.org/10.1099/00222615-48-4-357
De Vos D, Lim A Jr, Pirnay JP, Struelens M, Vandenvelde C, Duinslaeger L, Vanderkelen A, Cornelis P (1997) Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. J Clin Microbiol 35:1295–1299
Duan K, Lafontaine ER, Majumdar S, Sokol PA (2000) RegA, iron, and growth phase regulate expression of the Pseudomonas aeruginosa tol-oprL gene cluster. J Bacteriol 182:2077–2087. https://doi.org/10.1128/JB.182.8.2077-2087.2000
Locatelli L, Tarnawski S, Hamelin J, Rossi P, Aragno M, Fromin N (2002) Specific PCR amplification for the genus Pseudomonas targeting the 3ʹ half of 16S rDNA and the whole 16S–23S rDNA spacer. Syst Appl Microbiol 25:220–227. https://doi.org/10.1078/0723-2020-00110
Noike T, Endo G, Chang J, Yaguchi J, Matsumoto J (1985) Characteristics of carbohydrate degradation and the rate-limiting step in anaerobic digestion. Biotechnol Bioeng 27:1482–1489. https://doi.org/10.1002/bit.260271013
Baserba MG, Angelidaki I, Karakashev D (2012) Effect of continuous oleate addition on microbial communities involved in anaerobic digestion process. Bioresour Technol 106:74–81. https://doi.org/10.1016/j.biortech.2011.12.020
Gopinath LR, Merlin Christy P, Mahesh K, Bhuvaneswari R, Divya D (2014) Identification and evaluation of effective bacterial consortia for efficient biogas production. IOSR J Environ Sci Toxicol Food Technol 8:80–86
Resende JA, Silva VL, de Oliveira TLR, de Oliveira Fortunato S, da Costa Carneiro J, Otenio MH, Diniz CG (2014) Prevalence and persistence of potentially pathogenic and antibiotic resistant bacteria during anaerobic digestion treatment of cattle manure. Bioresour Technol 153:284–291. https://doi.org/10.1016/j.biortech.2013.12.007
Carlson CA, Ingraham JL (1983) Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl Environ Microbiol 45:1247–1253
Redfearn MS, Palleroni NJ, Stanier RY (1966) A comparative study of Pseudomonas pseudomallei and Bacillus mallei. J Gen Microbiol 43:293–313. https://doi.org/10.1099/00221287-43-2-293
Ali Shah F, Mahmood Q, Mahmood Shah M, Pervez A, Ahmad Asad S (2014) Microbial ecology of anaerobic digesters: the key players of anaerobiosis. Sci World J. https://doi.org/10.1155/2014/183752
Ramadan EM, AbdelHafez AA, Hassan EA, Saber FM (2016) Plant growth promoting rhizobacteria and their potential for biocontrol of phytopathogens. Afr J Microbiol Res 10:486–504. https://doi.org/10.5897/AJMR2015.7714
Jacoby GA (2006) β-Lactamase nomenclature. Antimicrob Agents Chemother 50:1123–1129. https://doi.org/10.1128/AAC.50.4.1123-1129.2006
Kuan KB, Othman R, Abdul Rahim K, Shamsuddin ZH (2016) Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS One. https://doi.org/10.1371/journal.pone.0152478
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Goswami D, Thakker JN, Dhandhukia PC (2016) Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric. https://doi.org/10.1080/23311932.2015.1127500
Maheshwari DK, Dheeman S, Agarwal M (2015) Phytohormone-producing PGPR for sustainable agriculture. In: Maheshwari DK (ed) Bacterial metabolites in sustainable agroecosystem. Springer, Cham, pp 159–182. https://doi.org/10.1007/978-3-319-24654-3_7
Qi G, Pan Z, Sugawa Y, Andriamanohiarisoamanana FJ, Yamashiro T, Iwasaki M, Kawamoto K, Ihara I, Umetsu K (2018) Comparative fertilizer properties of digestates from mesophilic and thermophilic anaerobic digestion of dairy manure: focusing on plant growth promoting bacteria (PGPB) and environmental risk. J Mater Cycles Waste Manag 20:1448–1457. https://doi.org/10.1007/s10163-018-0708-7
Pan Z, Qi G, Andriamanohiarisoamanana FJ, Yamashiro T, Iwasaki M, Nishida T, Tangtaweewipat S, Umetsu K (2018) Potential of anaerobic digestate of dairy manure in suppressing soil-borne plant disease. Anim Sci J. https://doi.org/10.1111/asj.13092
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (no. 10670499).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwasaki, M., Qi, G., Endo, Y. et al. Quantity changes in Pseudomonas species in dairy manure during anaerobic digestion at mesophilic and thermophilic temperatures. J Mater Cycles Waste Manag 21, 423–432 (2019). https://doi.org/10.1007/s10163-018-0800-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-018-0800-z