Abstract
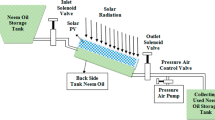
Pyrolysis of scrap tire using concentrated solar radiation is a novel way to upgrade feedstock. In this investigation, best operating condition for maximizing pyro-oil yield was determined. The parameters varied were the temperature of the reactor, flow rate of nitrogen gas and size of the feed particles. The maximum pyro-oil yield was 52 wt% at 400 °C of reactor temperature and nitrogen flow rate of 6 lpm for a feed size of 4 cm3. The pyro-oil and char were characterized according to ASTM standards. This research showed the feasibility of converting scrap tire into pyro-oil by using solar energy via pyrolysis and analysis showed the potential of pyro-oil and char as a valuable source of chemicals.
Graphical abstract







Similar content being viewed by others
References
Zeng K, Flamant G, Gauthier D, Guillot E (2015) Solar pyrolysis of wood in a lab-scale solar reactor: Influence of temperature and sweep gas flow rate on products distribution. Energy Proc 69:1849–1858. https://doi.org/10.1016/j.egypro.2015.03.163
Mondal S, Sharma AK, Sahoo PK (2014) Solar thermal biomass pyrolysis-a review paper. Int J Sci Eng Res 5:635–642
Hijazi H, Mokhiamar O, Elsamni O (2016) Mechanical design of a low cost parabolic solar dish concentrator. Alex Eng J 55:1–11. https://doi.org/10.1016/j.aej.2016.01.028
Islam MR, Tushar MSHK., Haniu H (2008) Production of liquid fuels and chemicals from pyrolysis of Bangladeshi bicycle/rickshaw tire wastes. J Anal Appl Pyrolysis 82:96–109. https://doi.org/10.1016/j.jaap.2008.02.005
Islam MR, Haniu H, Alam MR (2008) Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: product yields, compositions and related properties. Fuel 87:3112–3122. https://doi.org/10.1016/j.fuel.2008.04.036
Islam MR, Islam MN, Mustafi NN et al (2013) Thermal recycling of solid tire wastes for alternative liquid fuel: the first commercial step in Bangladesh. Proc Eng 56:573–582. https://doi.org/10.1016/j.proeng.2013.03.162
Demirbas A (2004) Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J Anal Appl Pyrolysis 72:243–248. https://doi.org/10.1016/j.jaap.2004.07.003
Tag AT, Duman G, Ucar S, Yanik J (2016) Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J Anal Appl Pyrolysis 120:200–206. https://doi.org/10.1016/j.jaap.2016.05.006
Quan C, Gao N, Song Q (2016) Pyrolysis of biomass components in a TGA and a fixed-bed reactor. J Anal Appl Pyrolysis 121:84–92. https://doi.org/10.1016/j.jaap.2016.07.005
Yanik J, Stahl R, Troeger N, Sinag A (2013) Pyrolysis of algal biomass. J Anal Appl Pyrolysis 103:134–141. https://doi.org/10.1016/j.jaap.2012.08.016
Li AM, Li XD, Li SQ et al (1999) Pyrolysis of solid waste in a rotary kiln: influence of final pyrolysis temperature on the pyrolysis products. J Anal Appl Pyrolysis 50:149–162. https://doi.org/10.1016/S0165-2370(99)00025-X
Chan WCR, Kelbon M, Krieger BB (1985) Modelling and experimental verification of physical and chemical processes during pyrolysis of a large biomass particle. Fuel 64:1505–1513. https://doi.org/10.1016/0016-2361(85)90364-3
Grassmann H, Boaro M (2015) Solar biomass pyrolysis with the linear mirror II. Smart Grid Renew Energy 6:179–186
Morales S, Miranda R, Bustos D et al (2014) Solar biomass pyrolysis for the production of bio-fuels and chemical commodities. J Anal Appl Pyrolysis 109:65–78. https://doi.org/10.1016/j.jaap.2014.07.012
Zeng K, Gauthier D, Minh DP et al (2017) Characterization of solar fuels obtained from beech wood solar pyrolysis. Fuel 188:285–293. https://doi.org/10.1016/j.fuel.2016.10.036
Zeng K, Gauthier D, Lu J, Flamant G (2015) Parametric study and process optimization for solar pyrolysis of beech wood. Energy Convers Manag 106:987–998. https://doi.org/10.1016/j.enconman.2015.10.039
Joardder MUH, Halder PK, Rahim A, Paul N (2014) Solar assisted fast pyrolysis: a novel approach of renewable energy production. J Eng 2014:9. https://doi.org/10.1155/2014/252848
Islam MN, Islam MN, Beg MRA, Islam MR (2005) Pyrolytic oil from fixed bed pyrolysis of municipal solid waste and its characterization. Renew Energy 30:413–420. https://doi.org/10.1016/j.renene.2004.05.002
Ly HV, Kim SS, Choi JH et al (2016) Fast pyrolysis of Saccharina japonica alga in a fixed-bed reactor for bio-oil production. Energy Convers Manag 122:526–534. https://doi.org/10.1016/j.enconman.2016.06.019
Choudhury N, Chutia R, Bhaskar T, Kataki R (2014) Pyrolysis of jute dust : effect of reaction parameters and analysis of products. J Mater Cycles Waste Manag 16:449–459. https://doi.org/10.1007/s10163-014-0268-4
Harman-Ware AE, Morgan T, Wilson M et al (2013) Microalgae as a renewable fuel source: fast pyrolysis of Scenedesmussp. Renew Energy 60:625–632. https://doi.org/10.1016/j.renene.2013.06.016
Islam MR, Joardder MUH, Hasan SM et al (2011) Feasibility study for thermal treatment of solid tire wastes in Bangladesh by using pyrolysis technology. Waste Manag 31:2142–2149. https://doi.org/10.1016/j.wasman.2011.04.017
Parveen M, Islam MR, Haniu H (2011) Thermal decomposition behavior study of two agricultural solid wastes for production of bio-fuels by pyrolysis technology. J Therm Sci Technol 6:132–139. https://doi.org/10.1299/jtst.6.132
Kader MA, Islam MR, Parveen M et al (2013) Pyrolysis decomposition of tamarind seed for alternative fuel. Bioresour Technol 149:1–7. https://doi.org/10.1016/j.biortech.2013.09.032
Ucar S, Karagoz S, Ozkan AR, Yanik J (2005) Evaluation of two different scrap tires as hydrocarbon source by pyrolysis. Fuel 84:1884–1892. https://doi.org/10.1016/j.fuel.2005.04.002
Santos BS, Capareda SC (2016) Energy sorghum pyrolysis using a pressurized batch reactor. Biomass Convers Biorefinery 6:325–334. https://doi.org/10.1007/s13399-015-0191-5
Piatkowski N, Wieckert C, Steinfeld A (2009) Experimental investigation of a packed-bed solar reactor for the steam-gasification of carbonaceous feedstocks. Fuel Process Technol 90:360–366. https://doi.org/10.1016/j.fuproc.2008.10.007
Daugaard DE, Brown RC (2003) Enthalpy for pyrolysis for several types of biomass. Energy Fuels 17:934–939. https://doi.org/10.1021/ef020260x
Chueh W, Falter C, Abbott M et al (2010) High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 330:1797–1801. https://doi.org/10.1126/science.1197834 doi
Rahman MA, Aziz MA, Ruhul AM, Rashid MM (2017) Biodiesel production process optimization from Spirulina maxima microalgae and performance investigation in a diesel engine. J Mech Sci Technol 31:3025–3033. https://doi.org/10.1007/s12206-017-0546-x
Rahman MA, Aziz MA, Al-khulaidi RA et al (2017) Biodiesel production from microalgae Spirulina maxima by two step process: optimization of process variable. J Radiat Res Appl Sci 10:140–147. https://doi.org/10.1016/j.jrras.2017.02.004
Nabi MN, Akhter MS, Rahman MA (2013) Waste transformer oil as an alternative fuel for diesel engine. Proc Eng 56:401–406. https://doi.org/10.1016/j.proeng.2013.03.139
Acknowledgements
Authors would like to acknowledge, Ministry of Power, Energy and Mineral Resources, Bangladesh for the partial financial support through research program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rahman, M.A., Aziz, M.A. Solar pyrolysis of scrap tire: optimization of operating parameters. J Mater Cycles Waste Manag 20, 1207–1215 (2018). https://doi.org/10.1007/s10163-017-0686-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-017-0686-1