Abstract
Purpose
To investigate the impact of rapamycin on the differentiation of hair cells.
Methods
Murine cochlear organoids were derived from cochlear progenitor cells. Different concentrations of rapamycin were added into the culture medium at different proliferation and differentiation stages.
Results
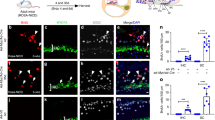
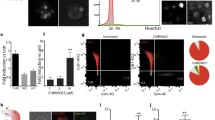
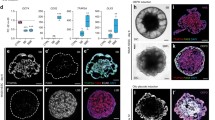
Rapamycin exhibited a concentration-dependent reduction in the proliferation of these inner ear organoids. Nevertheless, organoids subjected to a 10-nM dose of rapamycin demonstrated a markedly increased proportion of hair cells. Furthermore, rapamycin significantly upregulated the expression of markers associated with both hair cells and supporting cells, including ATOH1, MYO7A, and SOX2. Mechanistic studies revealed that rapamycin preferentially suppressed cells without Sox2 expression during the initial proliferation stage, thereby augmenting and refining the population of SOX2+ progenitors. These enriched progenitors were predisposed to differentiate into hair cells during the later stages of organoid development. Conversely, the use of the mTOR activator MHY 1485 demonstrated opposing effects.
Conclusion
Our findings underscore a practical strategy for enhancing the generation of inner ear organoids with a low dose of rapamycin, achieved by enriching SOX2+ progenitors in an in vitro setting.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author.
References
Shahab M, Rosati R, Meyer DN, Shields JN, Crofts E, Baker TR, Jamesdaniel S (2021) Cisplatin-induced hair cell loss in zebrafish neuromasts is accompanied by protein nitration and Lmo4 degradation. Toxicol Appl Pharmacol 410:115342. https://doi.org/10.1016/j.taap.2020.115342
Ding D, Allman BL, Salvi R (2012) Review: ototoxic characteristics of platinum antitumor drugs. Anat Rec (Hoboken) 295:1851–1867. https://doi.org/10.1002/ar.22577
Rubel EW, Furrer SA, Stone JS (2013) A brief history of hair cell regeneration research and speculations on the future. Hear Res 297:42–51. https://doi.org/10.1016/j.heares.2012.12.014
Corwin JT (1981) Postembryonic production and aging in inner ear hair cells in sharks. J Comp Neurol 201:541–553. https://doi.org/10.1002/cne.902010406
Corwin JT, Cotanche DA (1988) Regeneration of sensory hair cells after acoustic trauma. Science 240:1772–1774. https://doi.org/10.1126/science.3381100
Ryals BM, Rubel EW (1988) Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240:1774–1776. https://doi.org/10.1126/science.3381101
Burns JC, Stone JS (2017) Development and regeneration of vestibular hair cells in mammals. Semin Cell Dev Biol 65:96–105. https://doi.org/10.1016/j.semcdb.2016.11.001
Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, Chalasani K, Steigelman KA, Fang J, Rubel EW, Cheng AG, Zuo J (2014) Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 141:816–829. https://doi.org/10.1242/dev.103036
Weichhart T (2018) mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology 64:127–134. https://doi.org/10.1159/000484629
Sehgal SN, Baker H, Vézina C (1975) Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 28:727–732. https://doi.org/10.7164/antibiotics.28.727
Cortada M, Levano S, Bodmer D (2021) mTOR Signaling in the inner ear as potential target to treat hearing loss. Int J Mol Sci 22:6368. https://doi.org/10.3390/ijms22126368
Shu Y, Li W, Huang M, Quan YZ, Scheffer D, Tian C, Tao Y, Liu X, Hochedlinger K, Indzhykulian AA, Wang Z, Li H, Chen ZY (2019) Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat Commun 10:5530. https://doi.org/10.1038/s41467-019-13157-7
Li XJ, Doetzlhofer A (2020) LIN28B/let-7 control the ability of neonatal murine auditory supporting cells to generate hair cells through mTOR signaling. Proc Natl Acad Sci USA 117:22225–22236. https://doi.org/10.1073/pnas.2000417117
Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, Liu Z, Taketo MM, Oghalai JS, Nusse R, Zuo J, Cheng AG (2012) Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci USA 109:8167–8172. https://doi.org/10.1073/pnas.1202774109
McLean WJ, Yin X, Lu L, Lenz DR, McLean D, Langer R, Karp JM, Edge ASB (2017) Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep 18:1917–1929. https://doi.org/10.1016/j.celrep.2017.01.066
Lenz DR, Gunewardene N, Abdul-Aziz DE, Wang Q, Gibson TM, Edge ASB (2019) Applications of Lgr5-positive cochlear progenitors (LCPs) to the study of hair cell differentiation. Front Cell Dev Biol 7:14. https://doi.org/10.3389/fcell.2019.00014
Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE (2003) Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns 3:389–395. https://doi.org/10.1016/s1567-133x(03)00089-9
Roccio M, Edge ASB (2019) Inner ear organoids: new tools to understand neurosensory cell development, degeneration and regeneration. Dev 146
Kempfle JS, Turban JL, Edge AS (2016) Sox2 in the differentiation of cochlear progenitor cells. Sci Rep 6:23293. https://doi.org/10.1038/srep23293
Stojanova ZP, Kwan T, Segil N (2016) Epigenetic regulation of Atoh1 guides hair cell development in the mammalian cochlea. Dev 142:3529–3536. https://doi.org/10.1242/dev.137976
Yang LM, Cheah KSE, Huh SH, Ornitz DM (2019) Sox2 and FGF20 interact to regulate organ of Corti hair cell and supporting cell development in a spatially-graded manner. PLoS Genet 15:e1008254. https://doi.org/10.1371/journal.pgen.1008254
Gou Y, Vemaraju S, Sweet EM, Kwon HJ, Riley BB (2018) sox2 and sox3 play unique roles in development of hair cells and neurons in the zebrafish inner ear. Dev Biol 435:73–83. https://doi.org/10.1016/j.ydbio.2018.01.010
Yoo YJ, Kim H, Park SR, Yoon YJ (2017) An overview of rapamycin: from discovery to future perspectives. J Ind Microbiol Biotechnol 44:537–553. https://doi.org/10.1007/s10295-016-1834
Lu Q, Liu Y, Wang Y, Wang W, Yang Z, Li T, Tian Y, Chen P, Ma K, Jia Z, Zhou C (2017) Rapamycin efficiently promotes cardiac differentiation of mouse embryonic stem cells. Biosci Rep 37:BSR20160552. https://doi.org/10.1042/BSR20160552
Fang B, Xiao H (2014) Rapamycin alleviates cisplatin-induced ototoxicity in vivo. Biochem Biophys Res Commun 448:443–447. https://doi.org/10.1016/j.bbrc.2014.04.123
Kim YJ, Tian C, Kim J, Shin B, Choo OS, Kim YS, Choung YH (2017) Autophagic flux, a possible mechanism for delayed gentamicin-induced ototoxicity. Sci Rep 7:41356. https://doi.org/10.1038/srep41356
Yuan H, Wang X, Hill K, Chen J, Lemasters J, Yang SM, Sha SH (2015) Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal 22:1308–1324. https://doi.org/10.1089/ars.2014.6004
Altschuler RA, Kanicki A, Martin C, Kohrman DC, Miller RA (2018) Rapamycin but not acarbose decreases age-related loss of outer hair cells in the mouse cochlea. Hear Res 370:11–15. https://doi.org/10.1016/j.heares.2018.09.003
Fu X, Sun X, Zhang L, Jin Y, Chai R, Yang L, Zhang A, Liu X, Bai X, Li J, Wang H, Gao J (2018) Tuberous sclerosis complex-mediated mTORC1 overactivation promotes age-related hearing loss. J Clin Invest 128:4938–4955. https://doi.org/10.1172/JCI98058
Altschuler RA, Kabara L, Martin C, Kanicki A, Stewart CE, Kohrman DC, Dolan DF (2021) Rapamycin added to diet in late mid-life delays age-related hearing loss in UMHET4 mice. Front Cell Neurosci 15:658972. https://doi.org/10.3389/fncel.2021.658972
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293. https://doi.org/10.1016/j.cell.2012.03.017
Cracco JB, Serrano P, Moskowitz SI, Bergold PJ, Sacktor TC (2005) Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus 15:551–556. https://doi.org/10.1002/hipo.20078
Hosoya M, Fujioka M, Sone T, Okamoto S, Akamatsu W, Ukai H, Ueda HR, Ogawa K, Matsunaga T, Okano H (2017) Cochlear cell modeling using disease-specific iPSCs unveils a degenerative phenotype and suggests treatments for congenital progressive hearing loss. Cell Rep 18:68–81. https://doi.org/10.1016/j.celrep.2016.12.020
Hosoya M, Saeki T, Saegusa C, Matsunaga T, Okano H, Fujioka M, Ogawa K (2018) Estimating the concentration of therapeutic range using disease-specific iPS cells: low-dose rapamycin therapy for Pendred syndrome. Regen Ther 10:54–63. https://doi.org/10.1016/j.reth.2018.11.001
Qiu XX, Liu Y, Zhang YF, Guan YN, Jia QQ, Wang C, Liang H, Li YQ, Yang HT, Qin YW, Huang S, Zhao XX, Jing Q (2017) Rapamycin and chir99021 coordinate robust cardiomyocyte differentiation from human pluripotent stem cells via reducing p53-dependent apoptosis. J Am Heart Assoc 6:e005295. https://doi.org/10.1161/JAHA.116.005295
Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS (2005) Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434:1031–1035. https://doi.org/10.1038/nature03487
Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX (2012) Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell 22:377–390. https://doi.org/10.1016/j.devcel.2011.12.006
Neves J, Uchikawa M, Bigas A, Giraldez F (2012) The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS ONE 7:e30871. https://doi.org/10.1371/journal.pone.0030871
Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY (1999) Math1: an essential gene for the generation of inner ear hair cells. Science 284:1837–1841. https://doi.org/10.1126/science.284.5421.1837
Lewis RM, Hume CR, Stone JS (2012) Atoh1 expression and function during auditory hair cell regeneration in post-hatch chickens. Hear Res 289:74–85. https://doi.org/10.1016/j.heares.2012.04.008
Hicks KL, Wisner SR, Cox BC, Stone JS (2020) Atoh1 is required in supporting cells for regeneration of vestibular hair cells in adult mice. Hear Res 385:107838. https://doi.org/10.1016/j.heares.2019.107838
Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ (2007) Potential therapeutic applications of autophagy. Nat Rev Drug Discov 6:304–312. https://doi.org/10.1038/nrd2272
Reggiori F, Klionsky DJ (2002) Autophagy in the eukaryotic cell. Eukaryot Cell 1:11–21. https://doi.org/10.1128/EC.01.1.11-21.2002
Cho YH, Han KM, Kim D, Lee J, Lee SH, Choi KW, Kim J, Han YM (2014) Autophagy regulates homeostasis of pluripotency-associated proteins in hESCs. Stem Cells 32:424–435. https://doi.org/10.1002/stem.1589
Jia Z, Wang J, Wang W, Tian Y, XiangWei W, Chen P, Ma K, Zhou C (2014) Autophagy eliminates cytoplasmic β-catenin and NICD to promote the cardiac differentiation of P19CL6 cells. Cell Signal 26:2299–2305. https://doi.org/10.1016/j.cellsig.2014.07.028
Aburto MR, Sánchez-Calderón H, Hurlé JM, Varela-Nieto I, Magariños M (2012) Early otic development depends on autophagy for apoptotic cell clearance and neural differentiation. Cell Death Dis 3:e394. https://doi.org/10.1038/cddis.2012.132
He Z, Guo L, Shu Y, Fang Q, Zhou H, Liu Y, Liu D, Lu L, Zhang X, Ding X, Liu D, Tang M, Kong W, Sha S, Li H, Gao X, Chai R (2017) Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy 13:1884–1904. https://doi.org/10.1080/15548627.2017.1359449
Guo S, Xu N, Chen P, Liu Y, Qi X, Liu S, Li C, Tang J (2019) Rapamycin protects spiral ganglion neurons from gentamicin-induced degeneration in vitro. J Assoc Res Otolaryngol 20:475–487. https://doi.org/10.1007/s10162-019-00717-3
Acknowledgements
We are grateful to the National Natural Science Foundation for supporting this study.
Funding
This study was funded by the National Natural Science Foundation of China (82201280).
Author information
Authors and Affiliations
Contributions
W.W. and Y.L.: conceptualization, data curation, and formal analysis. W.W. and P.C.: investigation and validation. W.W.: original draft writing. J.Y.: supervision, review and editing. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, W., Chen, P., Yang, J. et al. A Low Dose of Rapamycin Promotes Hair Cell Differentiation by Enriching SOX2+ Progenitors in the Neonatal Mouse Inner Ear Organoids. JARO 25, 149–165 (2024). https://doi.org/10.1007/s10162-024-00938-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-024-00938-1