Abstract
Background
Tolvaptan, a vasopressin V2 receptor antagonist, is used to treat autosomal-dominant polycystic kidney disease (ADPKD). Although tolvaptan curbs disease progression, a few reports have examined factors related to treatment response. The estimated glomerular filtration rate (eGFR) decreases soon after tolvaptan is initiated. We investigated whether initial eGFR decline affects renal prognosis of patients.
Methods
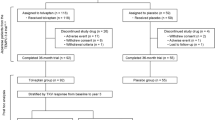
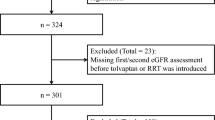
This was a single-center, retrospective observational cohort study. Eighty-three patients with ADPKD who initiated tolvaptan were selected. We analyzed the relationship of the initial eGFR change with clinical parameters and analyzed the annual eGFR change in terms of renal prognostic value using univariable and multivariable linear regression analyses.
Results
The initial eGFR change was − 4.6 ± 8.0%/month. The initial eGFR change correlated significantly with the annual eGFR change in multivariable analysis, suggesting that the larger decline in the initial eGFR change, the better the renal prognosis. Furthermore, the change in fractional excretion (FE) of free water (FEH2O) correlated positively with initial eGFR change. FEH2O and urea nitrogen FE (FEUN) increased significantly; however, sodium FE (FENa) level remained unchanged. In approximately half of the patients, FENa unexpectedly decreased.
Conclusions
The initial eGFR decline might be caused by suppressing glomerular hyperfiltration, due to the pharmacological effect of tolvaptan, and/or by reducing renal plasma flow, due to potential volume depletion. The initial eGFR change reflects the tolvaptan effect, can be easily evaluated in clinical practice, and may be useful as one of the clinical indicator for predicting renal prognosis in patients under tolvaptan.









Similar content being viewed by others
References
Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359(14):1477–85. https://doi.org/10.1056/NEJMcp0804458.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–18. https://doi.org/10.1056/NEJMoa1205511.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377(20):1930–42. https://doi.org/10.1056/NEJMoa1710030.
Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, et al. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol. 2018;29(10):2458–70. https://doi.org/10.1681/ASN.2018060590.
Reddy B, Chapman AB. Acute response to tolvaptan in ADPKD: a window to predict long-term efficacy? Am J Kidney Dis. 2015;65(6):811–3. https://doi.org/10.1053/j.ajkd.2015.03.004.
Irazabal MV, Torres VE, Hogan MC, Glockner J, King BF, Ofstie TG, et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80(3):295–301. https://doi.org/10.1038/ki.2011.119.
Boertien WE, Meijer E, de Jong PE, Bakker SJ, Czerwiec FS, Struck J, et al. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 2013;84(6):1278–86. https://doi.org/10.1038/ki.2013.285.
Boertien WE, Meijer E, de Jong PE, ter Horst GJ, Renken RJ, van der Jagt EJ, et al. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis. 2015;65(6):833–41. https://doi.org/10.1053/j.ajkd.2014.11.010.
Hansen HP, Rossing P, Tarnow L, Nielsen FS, Jensen BR, Parving HH. Increased glomerular filtration rate after withdrawal of long-term antihypertensive treatment in diabetic nephropathy. Kidney Int. 1995;47(6):1726–31. https://doi.org/10.1038/ki.1995.238.
Apperloo AJ, de Zeeuw D, de Jong PE. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int. 1997;51(3):793–7. https://doi.org/10.1038/ki.1997.111.
Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80(3):282–7. https://doi.org/10.1038/ki.2011.79.
Devuyst O, Chapman AB, Gansevoort RT, Higashihara E, Perrone RD, Torres VE, et al. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol. 2017;28(5):1592–602. https://doi.org/10.1681/ASN.2016040448.
Gansevoort RT, van Gastel MDA, Chapman AB, Blais JD, Czerwiec FS, Higashihara E, et al. Plasma copeptin levels predict disease progression and tolvaptan efficacy in autosomal dominant polycystic kidney disease. Kidney Int. 2019;96(1):159–69. https://doi.org/10.1016/j.kint.2018.11.044.
Makabe S, Manabe S, Kataoka H, Akihisa T, Yoshida R, Ushio Y, et al. Urinary Aquaporin 2 as a potential indicator predicting tolvaptan response in patients with ADPKD. Kidney Int Rep. 2021;6(9):2436–44. https://doi.org/10.1016/j.ekir.2021.06.033.
Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343(8901):824–7. https://doi.org/10.1016/s0140-6736(94)92026-5.
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Collaborators developing the Japanese equation for estimated GFR GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61(2):197–203. https://doi.org/10.1053/j.ajkd.2012.07.007.
Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–31. https://doi.org/10.1001/jama.2014.6634.
Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30(9):1735–45. https://doi.org/10.1681/ASN.2019010007.
Greene T, Ying J, Vonesh EF, Tighiouart H, Levey AS, Coresh J, et al. Performance of GFR slope as a surrogate end point for kidney disease progression in clinical trials: a statistical simulation. J Am Soc Nephrol. 2019;30(9):1756–69. https://doi.org/10.1681/ASN.2019010009.
Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Ren Physiol. 2005;288(5):F881–96. https://doi.org/10.1152/ajprenal.00367.2004.
Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol. 2013;9(4):223–39. https://doi.org/10.1038/nrneph.2013.22.
Bouby N, Ahloulay M, Nsegbe E, Dechaux M, Schmitt F, Bankir L. Vasopressin increases glomerular filtration rate in conscious rats through its antidiuretic action. J Am Soc Nephrol. 1996;7(6):842–51.
Zittema D, Boertien WE, van Beek AP, Dullaart RP, Franssen CF, de Jong PE, et al. Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin J Am Soc Nephrol. 2012;7(6):906–13. https://doi.org/10.2215/CJN.11311111.
Bankir L, Bichet DG. What can copeptin tell us in patients with autosomal dominant polycystic disease? Kidney Int. 2019;96(1):19–22. https://doi.org/10.1016/j.kint.2019.02.037.
Minami S, Hamano T, Iwatani H, Mizui M, Kimura Y, Isaka Y. Tolvaptan promotes urinary excretion of sodium and urea: a retrospective cohort study. Clin Exp Nephrol. 2018;22(3):550–61. https://doi.org/10.1007/s10157-017-1475-9.
Kajimoto K, Abe T. Blood urea nitrogen as a marker of the acute response to addition of tolvaptan to standard therapy in patients hospitalized for acute heart failure syndromes. Int J Cardiol. 2014;177(2):589–91. https://doi.org/10.1016/j.ijcard.2014.08.147.
Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG. Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol. 2005;16(7):1920–8. https://doi.org/10.1681/ASN.2004121079.
Perucca J, Bichet DG, Bardoux P, Bouby N, Bankir L. Sodium excretion in response to vasopressin and selective vasopressin receptor antagonists. J Am Soc Nephrol. 2008;19(9):1721–31. https://doi.org/10.1681/ASN.2008010021.
Nowson CA, Lim K, Campbell NRC, O’Connell SL, He FJ, Daly RM. Impact of fractional excretion of sodium on a single morning void urine collection as an estimate of 24-hour urine sodium. J Clin Hypertens (Greenwich). 2019;21(12):1763–70. https://doi.org/10.1111/jch.13725.
Kramers BJ, van Gastel MDA, Boertien WE, Meijer E, Gansevoort RT. Determinants of urine volume in ADPKD patients using the vasopressin V2 receptor antagonist tolvaptan. Am J Kidney Dis. 2019;73(3):354–62. https://doi.org/10.1053/j.ajkd.2018.09.016.
Cote G, Asselin-Thompstone L, Mac-Way F, Rene de Cotret P, Lacroix C, Desmeules S, et al. Sodium and urea excretion as determinants of urine output in autosomal dominant polycystic kidney disease patients on V2 receptor antagonists: impact of dietary intervention. Int Urol Nephrol. 2020;52(2):343–9. https://doi.org/10.1007/s11255-020-02384-3.
Gansevoort RT, Meijer E, Chapman AB, Czerwiec FS, Devuyst O, Grantham JJ, et al. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 Trial. Nephrol Dial Transplant. 2016;31(11):1887–94. https://doi.org/10.1093/ndt/gfv422.
Acknowledgements
We express our sincere appreciation to all the patients, collaborating physicians, and other medical staff for their important contributions to the study. This study was supported in part by a Grant-in-Aid for Intractable Renal Diseases Research, Research on rare and intractable diseases, Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Toshio Mochizuki received honoraria for lectures from Otsuka Pharmaceutical Co. Toshio Mochizuki and Hiroshi Kataoka belong to an endowed department sponsored by Otsuka Pharmaceutical Co, Chugai Pharmaceutical Co, Kyowa Kirin Co, and JMS Co.
Ethical approval
All procedures involving human participants were performed in accordance with the ethical standards of the institutional research committee of Tokyo Women’s Medical University (IRB approval number 5118) and with the tenets of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Akihisa, T., Kataoka, H., Makabe, S. et al. Initial decline in eGFR to predict tolvaptan response in autosomal-dominant polycystic kidney disease. Clin Exp Nephrol 26, 540–551 (2022). https://doi.org/10.1007/s10157-022-02192-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02192-2