Abstract
Background
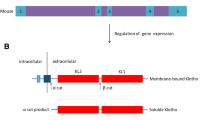
Soluble Klotho (sKl), the free form of membrane-bound Klotho predominantly expressed in the kidney, is detectable in serum and may have multiple pleiotropic effects. Patients with end-stage kidney disease are possibly sKl deficient, and kidney transplantation is the treatment of choice in these patients; however, little is known about changes in posttransplant sKl level and the factors influencing these changes.
Methods
We conducted a prospective longitudinal study to examine changes in posttransplant sKl level in recipients for 12 months after living-donor kidney transplantation and analyzed correlations between posttransplant changes in sKl levels and various influencing factors in both recipients and donors.
Results
29 kidney transplant recipients and their living donors were included for analysis. The results showed that sKl levels transiently decreased at 1 week posttransplant but progressively increased thereafter for 12 months. Multivariable linear regression analysis showed that body surface area-adjusted donor sKl levels were associated with posttransplant increases in recipient sKl levels at 12 months. In addition, pretransplant recipient sKl levels and body surface area-adjusted donor sKl levels were identified as an independent predictor of 12-month posttransplant sKl levels.
Conclusion
Pretransplant sKl levels in both kidney recipients and living donors are a strong determinant of sKl levels after kidney transplantation.



Similar content being viewed by others
References
Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51.
Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–33.
Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3.
Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4.
Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–801.
Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for alpha-, beta-, and gamma-secretase. FEBS Lett. 2009;583:3221–4.
Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–7.
Chen CD, Tung TY, Liang J, et al. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry. 2014;53:5579–87.
Mencke R, Olauson H, Hillebrands JL. Effects of Klotho on fibrosis and cancer: a renal focus on mechanism and therapeutic strategies. Adv Drug Deliv Rev. 2017;121:85–100.
Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol. 2012;8:423–9.
Sakan H, Nakatani K, Asai O, et al. Reduced renal alpha-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One. 2014;9:e86301.
Hirukawa T, Kakuta T, Nakamura M, et al. Mineral and bone disorders in kidney transplant recipients: reversible, irreversible, and de novo abnormalities. Clin Exp Nephrol. 2015;19:543–55.
Tonelli M, Wiebe N, Knoli G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–109.
Tan SJ, Crosthwaite A, Langsford D, et al. Mineral adaptations following kidney transplantation. Transpl Int. 2017;30:463–73.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Man L, Tahhan HR. Body surface area: a predictor of response to red blood cell transfusion. J Blood Med. 2016;7:199–204.
Malyszko J, Koc-Zorawska E, Matuszkiewicz-Rowinska J, Malyszko J. FGF23 and Klotho in relation to markers of endothelial dysfunction in kidney transplant recipients. Transplant Proc. 2014;46:2647–50.
Bleskestad IH, Thorsen IS, Jonsson G, Skadberg Ø, Bergrem H, Gøransson LG. Soluble Klotho and intact fibroblast growth factor 23 in long-term kidney transplant patients. Eur J Endocrinol. 2015;172:343–50.
Leone F, Lofaro D, Gigliotti P, et al. Soluble Klotho levels in adult renal transplant recipients are modulated by recombinant human erythropoietin. J Nephrol. 2014;27:577–85.
Akimoto T, Kimura T, Watanabe Y, et al. The impact of nephrectomy and renal transplantation on serum levels of soluble Klotho protein. Transplant Proc. 2013;45:134–6.
Hu MC, Shi M, Zhang J, et al. Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol. 2016;27:79–90.
Kimura T, Akimoto T, Watanabe Y, et al. Impact of renal transplantation and nephrectomy on urinary soluble Klotho protein. Transplant Proc. 2015;47:1697–9.
Qian Y, Guo X, Che L, et al. Klotho reduces necroptosis by targeting oxidative stress involved in renal ischemic-reperfusion injury. Cell Physiol Biochem. 2018;45:2268–82.
Yoon HE, Ghee JY, Piao S, et al. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant. 2011;26:800–13.
Han DH, Piao SG, Song JH, et al. Effect of sirolimus on calcineurin inhibitor-induced nephrotoxicity using renal expression of Klotho, an anti-aging gene. Transplantation. 2010;90:135–41.
Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81:640–50.
Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25:2169–75.
Takahashi H, Komaba H, Takahashi Y, et al. Impact of parathyroidectomy on serum FGF23 and soluble Klotho in hemodialysis patients with severe secondary hyperparathyroidism. J Clin Endocrinol Metab. 2014;99:E652–8.
Vo HT, Laszczyk AM, King GD. Klotho, the key to healthy brain aging? Brain Plast. 2018;3:183–94.
Imura A, Tsuji Y, Murata M, et al. αKlotho as a regulator of calcium homeostasis. Science. 2007;316:1615–8.
Sato T, Komaba H, Nagatani T, Watanabe T, Kishida Y, Fukagawa M. The pituitary is a candidate organ that modulates circulating Klotho levels. J Endocr Soc. 2018;3:52–61.
Castellano G, Intini A, Stasi A, et al. Complement modulation of anti-aging factor klotho in ischemia/reperfusion injury and delayed graft function. Am J Transplant. 2016;16:325–33.
Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol. 2012;303:F1641–51.
Rakugi H, Matsukawa N, Ishikawa K, et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82–7.
Adema AY, Vervloet MG, Blankenstein MA, Heijboer AC. α-Klotho is unstable in human urine. Kidney Int. 2015;88:1442–4.
Acknowledgements
We thank Chigusa Ishioka for her help with sample measurement.
Funding
This work was supported in part by JSPS KAKENHI (grant no. JP 25461962) and also by a grant from The Kidney Foundation, Japan (no. JKFB18-29 to Dr. Nakamura). The founder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. We also thank the Tokai University General Research Organization for funding the editing and proofreading of this paper.
Author information
Authors and Affiliations
Contributions
HI and MN designed the study. HI analyzed the data. HI, MN, SU, ST, HK, and MF performed the study. HI wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Nakamura received grant support from Astellas Pharma and Novartis. Dr. Komaba has received honoraria, consulting fees, and/or grant support from Bayer Yakuhin, Chugai Pharmaceutical, Japan Tobacco, Kyowa Kirin, Novartis, and Ono Pharmaceutical. Dr. Fukagawa has received honoraria, consulting fees, and/or grant support from Bayer Yakuhin, Chugai Pharmaceutical, Fresenius Kabi, Kissei Pharmaceutical, Kyowa Kirin, Ono Pharmaceutical, and Torii Pharmaceutical. The remaining authors declare no competing interests.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB approval number 13R015) and with the 1964 Helsinki declaration and its later amendments of comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ishida, H., Nakamura, M., Komaba, H. et al. Post-kidney transplant soluble Klotho levels are determined by pretransplant soluble Klotho levels in both living donors and recipients. Clin Exp Nephrol 25, 1367–1374 (2021). https://doi.org/10.1007/s10157-021-02112-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-021-02112-w