Abstract
Background
Recent studies have suggested that erythropoiesis-stimulating agents (ESAs) may accelerate not only angiogenesis but also vasculogenesis, beyond erythropoiesis.
Methods
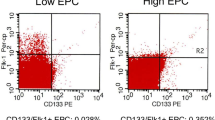
We conducted a 12-week prospective study in 51 dialysis patients; 13 were treated with recombinant human erythropoietin (EPO, 5290.4 ± 586.9 IU/week), 16 with darbepoetin (DA, 42.9 ± 4.3 µg/week), 12 with epoetin β pegol (CERA, 40.5 ± 4.1 µg/week) and 10 with no ESAs. Vascular mediators comprising endothelial progenitor cells (EPCs), vascular endothelial growth factor (VEGF), matrix metalloproteinase-2 (MMP-2), and high-sensitivity C-reactive protein (hs-CRP) were measured at 0 and 12 weeks. EPCs were measured by flow cytometry as CD45lowCD34+CD133+ cells.
Results
The EPC count increased significantly to a greater extent in the EPO group than in the other three group, and increased significantly from 0 to 12 weeks in a EPO dose-dependent manner. In both the DA and CERA groups, the EPC count did not change at 12 weeks. Serum levels of VEGF, MMP-2 and hs-CRP were not affected by ESA treatment in all groups. In the CERA group, serum ferritin decreased significantly compared to the no-ESA group and correlated with CERA dose, although use of iron was permitted if required during the prospective study period of 12 weeks.
Conclusions
When patients on dialysis were treated with clinical doses of various ESAs, only EPO induced a significant increase of circulating EPCs from bone marrow, whereas, DA and CERA had no effect.

Similar content being viewed by others
Change history
05 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10157-021-02103-x
16 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10157-021-02162-0
References
Eguchi M, Masuda H, Asahara T. Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp Nephrol. 2007;11:18–25.
Chateauvieux S, Grigorakaki C, Morceau F, Dicato M, Diederich M. Erythropoietin, erythropoiesis and beyond. Biochem Pharmacol. 2011;82:1291–303.
Ribatti D, Vacca A, Roccaro AM, Crivellato E, Presta M. Erythropoietin as an angiogenetic factor. Eur J Clin Invest. 2003;33:891–6.
Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenetic potential. Microvasc Res. 2002;64:326–323.
Heeschen C, Aicher A, Lehmann R, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–6.
Bahlmann FH, de Groot K, Spandau JM, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–6.
Naito T, Sanaka T, Mikami H, et al. Modulation of circulating endothelial progenitor cells by erythropoiesis-stimulating agents in patients with chronic kidney disease stage G5 and 5D. Clin Nephrol. 2016;86:242–52.
Leshem-Rubinow E, Steinvil A, Zeltser D, et al. Association of angiotensin-converting enzyme inhibitor therapy initiation with a reduction in hemoglobin levels in patients without renal failure. Mayo Clin Proc. 2012;87:1189–95.
Le Meur Y, Lorgeot V, Comte L, et al. Plasma levels and metabolism of AcSDKP in patients with chronic renal failure: relationship with erythropoietin requirements. Am J Kidney Dis. 2001;38:510–7.
Matsusaka S, Mishima Y, Suenaga M, et al. Circulating endothelial progenitors and CXCR4-positive circulating endothelial cells are predictive markers for bevacizumab. Cancer. 2011;117:4026–32.
Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–9.
Locatelli F, Andrulli S, Memoli B, et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant. 2006;21:991–8.
Morikami Y, Fujimori A, Okada S, Kumei M, Mizobuchi N, Sakai M. Twice-monthly administration of a lower dose of epoetin beta pegol can maintain adequate hemoglobin levels in hemodialysis patients. Ther Apher Dial. 2015;19:138–43.
Solomon A, Blum A, Peleg A, Lev El, Leshem-Lev D, Hasin Y. Endothelial progenitor cells are suppressed in anemic patients with acute coronary syndrome. Am J Med 2012;125:604–611.
Sturiale A, Coppolino G, Loddo S, et al. Effects of haemodialysis on circulating endothelial progenitor cell count. Blood Purif. 2007;25:242–51.
Georgescu A, Alexandru N, Andrei E, et al. Circulating microparticles and endothelial progenitor cells in atherosclerosis: pharmacological effects of irbesartan. J Thromb Haemost. 2012;10:680–91.
Saito H, Yamamoto Y, Yamamoto H. Diabetes alters subsets of endothelial progenitor cells that reside in blood, bone marrow, and spleen. Am J Physiol Cell Physiol. 2012;302:C892-901.
Xia WH, Yang Z, Xu SY, et al. Age-related decline in reendothelialization capacity of human endothelial progenitor cells is restored by shear stress. Hypertension. 2012;59:1225–31.
Yang JX, Tang WL, Wang XX. Superparamagnetic iron oxide nanoparticles may affect endothelial progenitor cell migration ability and adhesion capacity. Cytotherapy. 2010;12:251–9.
Elliott S, Sinclair AM. The effect of erythropoietin on normal and neoplastic cells. Biologics. 2012;6:163–89.
Kawai T, Kusano Y, Yamada K, Ueda C, Kawai A, Takao M. Long-term maintenance of hemoglobin levels in hemodialysis patients treated with bi-weekly epoetin beta pegol switched from darbepoetin alfa: a single-center, 12-month observational study in Japan. J Artif Organs. 2019;22:146–53.
Brines M, Cerami A. Discovering erythropoietin’s extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246–50.
Sinclair AM, Coxon A, McCaffery I, et al. Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood. 2010;115:4264–72.
Brines M, Grasso G, Fiordaliso F, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907–12.
Zhang Z, Yao L, Yang J, Wang Z, Du G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia. Mol Med Rep. 2018;18:3547–54.
Westenbrink BD, Lipsic E, van der Meer P, et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–27.
Cheng Y, Hu R, Lv L, Ling L, Jiang S. Erythropoietin improves the efficiency of endothelial progenitor cell therapy after myocardial infarction in mice: effects on transplanted cell survival and autologous endothelial progenitor cell mobilization. J Surg Res. 2012;176:e47-55.
Hand CC, Brines M. Promises and pitfalls in erythropoietin-mediated tissue protection: are nonerythropoietic derivatives a way forward? J Investig Med. 2011;59:1073–82.
Povsic TJ, Najjar SS, Prather K, et al. EPC mobilization after erythropoietin treatment in acute ST-elevation myocardial infarction: the REVEAL EPC substudy. J Thromb Thrombolysis. 2013;36:375–83.
Bahlmann FH, DeGroot K, Duckert T, et al. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 2003;64:1648–52.
Shimoni S, Bar I, Meledin V, Derazne E, Gandelman G, George J. Circulating endothelial progenitor cells and clinical outcome in patients with aortic stenosis. PLoS ONE. 2016;11:e0148766. https://doi.org/10.1371/journal.pone.0148766.
Krenning G, Dankers PY, Drouven JW, et al. Endothelial progenitor cell dysfunction in patients with progressive chronic kidney disease. Am J Physiol Renal Physiol. 2009;296:F1314–22.
Kiss Z, Elliot S, Jedynasty K, Tesar V, Szegedi J. Discovery and basic pharmacology of erythropoiesis-stimulating agents (ESAs), including the hyperglycosylated ESA, darbepoetin alfa: an update of the rationale and clinical impact. Eur J Clin Pharmacol. 2010;66:331–40.
Jarsch M, Brandt M, Lanzendorfer M, Haselbeck A. Comparative erythropoietin receptor binding kinetics of CERA and epoetin-beta determined by surface plasmon resonance and competition binding assay. Pharmacology. 2008;81:63–9.
Ribatti D. The discovery of endothelial progenitor cells. An historical review. Leuk Res. 2007;31:439–44.
Basak GW, Yasukawa S, Alfaro A, et al. Human embryonic stem cells hemangioblast express HLA-antigens. J Transl Med. 2009;7:27. https://doi.org/10.1186/1479-5876-7-27.
Mohler ER 3rd, Zhang L, Medenilla E, et al. Effect of darbepoetin alfa on endothelial progenitor cells and vascular reactivity in chronic kidney disease. Vasc Med. 2011;16:183–9.
Sakaguchi Y, Hamano T, Wada A, Masakane I. Types of erythropoietin-stimulating agents and mortality among patients undergoing hemodialysis. J Am Soc Nephrol. 2019;30:1037–48.
Onuma S, Honda H, Kobayashi Y, et al. Effects of long-term erythropoiesis-stimulating agents on iron metabolism in patients on hemodialysis. Ther Apher Dial. 2015;19:582–9.
Takasawa K, Takaeda C, Maeda T, Ueda N. Hepcidin-25, mean corpuscular volume, and ferritin as predictors of response to oral iron supplementation in hemodialysis patients. Nutrients. 2014;7:103–18.
Maruyama Y, Yokoyama K, Yokoo T, Shigematsu T, Iseki K, Tsubakihara Y. The different association between serum ferritin and mortality in hemodialysis and peritoneal dialysis patients using Japanese nationwide dialysis registry. PLoS ONE. 2015;10:e0143430. https://doi.org/10.1371/journal.pone.0143430.
Trincavelli ML, Da Pozzo E, Ciampi O, et al. Regulation of erythropoietin receptor activity in endothelial cells by different erythropoietin (EPO) derivatives: an in vitro study. Int J Mol Sci. 2013;14:2258–81.
Westenbrink BD, Ruifrok WP, Voors AA, et al. Vascular endothelial growth factor is crucial for erythropoietin-induced improvement of cardiac function in heart failure. Cardiovasc Res. 2010;87:30–9.
Li Y, Ogle ME, Wallace GC IV, Lu ZY, Yu SP, Wei L. Erythropoietin attenuates intracerebral hemorrhage by diminishing matrix metalloproteinases and maintaining blood-brain barrier integrity in mice. Acta Neurochir Suppl. 2008;105:105–12.
Yeh KH, Tsai TH, Chai HT, et al. Comparison of acute versus convalescent stage high-sensitivity C-reactive protein level in predicting clinical outcome after acute ischemic stroke and impact of erythropoietin. J Transl Med. 2012;10:6. https://doi.org/10.1186/1479-5876-10-6.
Vacek TP, Rehman S, Neamtu D, Yu S, Givimani S, Tyagi SC. Matrix metalloproteinases in atherosclerosis: role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc Health Risk Manag. 2015;11:173–83.
Chan CY, Chen YS, Lee HH, et al. Erythropoietin protects post-ischemic hearts by preventing extracellular matrix degradation: role of Jak2-ERK pathway. Life Sci. 2007;81:717–23.
Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–32.
Funding
This study was supported in part by grants from the Kidney Foundation of Japan (grant number JKFB 17-7 (2017–2019)), and also by research funds from the Japanese Ministry of Health, Labor and Welfare (2015–2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
The study was a conducted in accordance with the Declaration of Helsinki and was approved by the independent Ethics Committee of Tokyo Women’s Medical University (No. 1135) and Tokyo Rosai Hospital (No. 2414).
Informed consent
Informed consent was obtained from all individuals participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Naito, T., Shun, M., Nishimura, H. et al. Pleiotropic effect of erythropoiesis-stimulating agents on circulating endothelial progenitor cells in dialysis patients. Clin Exp Nephrol 25, 1111–1120 (2021). https://doi.org/10.1007/s10157-021-02071-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-021-02071-2