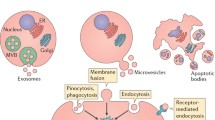
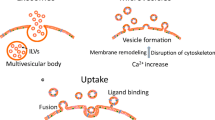
Abstract
MiRNAs play essential roles in processes of physiological status and disease conditions including in renal diseases, while extracellular vesicles (EVs) serve as important mediators for cell–cell communication. In body fluid or extracellular spaces, miRNAs are packaged into EVs and transferred to targeted cells to perform their bioeffects under particular conditions. In the present review, we aim to summarize and update the known and verified EV-carrying miRNAs (EV-miRNAs) and their general roles in kidney diseases. In addition to performing a systemic analysis, we try to provide some clues and perspectives for the future study of EV-miRNAs in renal diseases.




Similar content being viewed by others
Abbreviations
- AKI:
-
Acute kidney injury
- IgAN:
-
IgA Nephropathy
- FSGS/MCD:
-
Focal segmental glomerulosclerosis/minimal change disease (nephropathy)
- CKD:
-
Chronic kidney disease
- DN:
-
Diabetic nephropathy
- DKD:
-
Diabetic kidney disease
- LN:
-
Lupus nephritis
- DM:
-
Diabetes mellitus
- MA:
-
Albuminuri
- HG:
-
High glucos
- EMP:
-
Endothelial microparticle
- MIC/MAC:
-
Microalbuminuria/macroabuminuria
- NGT:
-
Normal glucose
- EH:
-
Essential hypertension
- RVH:
-
Renovascular hypertension
- ccRCC:
-
Clear cell renal cell carcinoma
- RAPC:
-
Renal artery-derived vascular progenitor cells
- hWJMSC:
-
Human Wharton jelly mesenchymal stromal cells
- ECFC:
-
Human endothelial colony forming cell
- BM:
-
Bone marrow
- MSC:
-
Mesenchymal stromal cells
- HLSC:
-
Human liver stem cell
- MC:
-
Mesangial cells
- UUO:
-
Unilateral ureteral occlusion
- IRI:
-
Ischemia–reperfusion injury
- EPO:
-
Erythropoietin
- MV:
-
Microvesicles
- HCAEC:
-
Human coronary artery endothelial cells
References
Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2018;19(12):808.
Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circ Res. 2017;120(2):381–99.
Godlewski J, et al. Belonging to a network–microRNAs, extracellular vesicles, and the glioblastoma microenvironment. Neuro Oncol. 2015;17(5):652–62.
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Zhang W, et al. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol. 2016;311(5):F844–F851851.
Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Deregibus MC, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–8.
Salvi V, et al. Exosome-delivered microRNAs promote IFN-alpha secretion by human plasmacytoid DCs via TLR7. JCI Insight. 2018;3(10):e98204.
Zhang Y, et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548(7665):52–7.
Vinas JL, et al. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int. 2016;90(6):1238–50.
Yanez-Mo M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
Shurtleff MJ, et al. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276.
Bitzer M, Ben-Dov IZ, Thum T. Microparticles and microRNAs of endothelial progenitor cells ameliorate acute kidney injury. Kidney Int. 2012;82(4):375–7.
Gracia T, et al. Urinary exosomes contain microRNAs capable of paracrine modulation of tubular transporters in kidney. Sci Rep. 2017;7:40601.
Rossol-Allison J, Ward CJ. Exosomes to the Rescue. J Am Soc Nephrol. 2015;26(10):2303–4.
Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–21.
Hoste E, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–25.
Collino F, et al. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNAs. J Am Soc Nephrol. 2015;26(10):2349–60.
Ichii O, et al. Altered expression of microRNA miR-146a correlates with the development of chronic renal inflammation. Kidney Int. 2012;81(3):280–92.
Cho YE, et al. Circulating plasma and exosomal micrornas as indicators of drug-induced organ injury in rodent models. Biomol Ther (Seoul). 2017;25(4):367–73.
Jia P, et al. MicroRNA-21 is required for local and remote ischemic preconditioning in multiple organ protection against sepsis. Crit Care Med. 2017;45(7):e703–e710710.
Lindoso RS, et al. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. 2014;23(15):1809–19.
Pang P, et al. Human vascular progenitor cells derived from renal arteries are endothelial-like and assist in the repair of injured renal capillary networks. Kidney Int. 2017;91(1):129–43.
Gu D, et al. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016;2016:2093940.
Cantaluppi V, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82(4):412–27.
Romagnani P, et al. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088.
Rodrigues JC, Haas M, Reich HN. IgA Nephropathy. Clin J Am Soc Nephrol. 2017;12(4):677–86.
Min QH, et al. Differential expression of urinary exosomal microRNAs in IgA nephropathy. J Clin Lab Anal. 2018;32(2):e22226.
Duan ZY, et al. Selection of urinary sediment miRNAs as specific biomarkers of IgA nephropathy. Sci Rep. 2016;6:23498.
Ramezani A, et al. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur J Clin Invest. 2015;45(4):394–404.
Huang Z, et al. Urinary exosomal miR-193a can be a potential biomarker for the diagnosis of primary focal segmental glomerulosclerosis in children. Biomed Res Int. 2017;2017:7298160.
Khurana R, et al. Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA. 2017;23(2):142–52.
Lv LL, et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305(8):F1220–F12271227.
Yu Y, et al. Non-proximal renal tubule-derived urinary exosomal miR-200b as a biomarker of renal fibrosis. Nephron. 2018;139(3):269–82.
Xie J, et al. The relationship between amniotic fluid miRNAs and congenital obstructive nephropathy. Am J Transl Res. 2017;9(4):1754–63.
Xie JX, et al. MicroRNA profiling in kidney disease: plasma versus plasma-derived exosomes. Gene. 2017;627:1–8.
Zhou Y, et al. Secreted fibroblast-derived miR-34a induces tubular cell apoptosis in fibrotic kidney. J Cell Sci. 2014;127(Pt 20):4494–506.
Wang X, et al. Unique molecular profile of exosomes derived from primary human proximal tubular epithelial cells under diseased conditions. J Extracell Vesicles. 2017;6(1):1314073.
Ichii O, et al. Urinary exosome-derived microRNAs reflecting the changes of renal function and histopathology in dogs. Sci Rep. 2017;7:40340.
Zhou Y, et al. Erythropoietin protects the tubular basement membrane by promoting the bone marrow to release extracellular vesicles containing tPA-targeting miR-144. Am J Physiol Renal Physiol. 2016;310(1):F27–40.
Wang B, et al. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol Ther. 2016;24(7):1290–301.
Wang Y, et al. Differentially expressed microRNAs in bone marrow mesenchymal stem cell-derived microvesicles in young and older rats and their effect on tumor growth factor-beta1-mediated epithelial-mesenchymal transition in HK2 cells. Stem Cell Res Ther. 2015;6:185.
He J, et al. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology (Carlton). 2015;20(9):591–600.
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45.
Jansen F, et al. Vascular endothelial microparticles-incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc Diabetol. 2016;15:49.
Zheng Z, et al. The coordinated roles of miR-26a and miR-30c in regulating TGFbeta1-induced epithelial-to-mesenchymal transition in diabetic nephropathy. Sci Rep. 2016;6:37492.
Eissa S, Matboli M, Bekhet MM. Clinical verification of a novel urinary microRNA panel: 133b, -342 and -30 as biomarkers for diabetic nephropathy identified by bioinformatics analysis. Biomed Pharmacother. 2016;83:92–9.
Jia Y, et al. miRNAs in Urine Extracellular Vesicles as Predictors of Early-Stage Diabetic Nephropathy. J Diabetes Res. 2016;2016:7932765.
Jia Y, et al. Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp Mol Med. 2018;50(5):56.
Xie Y, et al. Urinary Exosomal MicroRNA Profiling in Incipient Type 2 Diabetic Kidney Disease. J Diabetes Res. 2017;2017:6978984.
Eissa S, et al. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J Diabetes Complications. 2016;30(8):1585–92.
Delic D, et al. Urinary Exosomal miRNA Signature in Type II Diabetic Nephropathy Patients. PLoS ONE. 2016;11(3):e0150154.
Prabu P, et al. MicroRNAs from urinary extracellular vesicles are non-invasive early biomarkers of diabetic nephropathy in type 2 diabetes patients with the 'Asian Indian phenotype'. Diabetes Metab. 2018;45(3):276–85.
Ghai V, et al. Genome-wide profiling of urinary extracellular vesicle microRNAs associated with diabetic nephropathy in type 1 diabetes. Kidney Int Rep. 2018;3(3):555–72.
Jia Y, et al. MiR-4756 promotes albumin-induced renal tubular epithelial cell epithelial-to-mesenchymal transition and endoplasmic reticulum stress via targeting Sestrin2. J Cell Physiol. 2019;234(3):2905–15.
Mohan A, et al. Urinary exosomal microRNA-451-5p is a potential early biomarker of diabetic nephropathy in rats. PLoS ONE. 2016;11(4):e0154055.
Gallo S, et al. Stem cell-derived, microrna-carrying extracellular vesicles: a novel approach to interfering with mesangial cell collagen production in a hyperglycaemic setting. PLoS ONE. 2016;11(9):e0162417.
Yu F, et al. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol. 2017;13(8):483–95.
Sole C, et al. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol Dial Transplant. 2015;30(9):1488–96.
Perez-Hernandez J, et al. Increased urinary exosomal microRNAs in patients with systemic lupus erythematosus. PLoS ONE. 2015;10(9):e0138618.
Tangtanatakul P, et al. Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pac J Allergy Immunol. 2018;37(4):189–97.
Ichii O, et al. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS ONE. 2014;9(10):e110383.
Kwon SH, et al. Differential expression of microRNAs in urinary extracellular vesicles obtained from hypertensive patients. Am J Kidney Dis. 2016;68(2):331–2.
Perez-Hernandez J, et al. Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. J Transl Med. 2018;16(1):228.
Kim MH, et al. Urinary exosomal viral microRNA as a marker of BK virus nephropathy in kidney transplant recipients. PLoS ONE. 2017;12(12):e0190068.
Butz H, et al. Exosomal microRNAs are diagnostic biomarkers and can mediate cell-cell communication in renal cell carcinoma. Eur Urol Focus. 2016;2(2):210–8.
Zhang W, et al. MicroRNAs in serum exosomes as potential biomarkers in clear-cell renal cell carcinoma. Eur Urol Focus. 2016;4(3):412–9.
Zhou X, et al. Enhancer of zeste homolog 2 inhibition attenuates renal fibrosis by maintaining smad7 and phosphatase and tensin homolog expression. J Am Soc Nephrol. 2016;27(7):2092–108.
Luo J, et al. Serum glucocorticoid-regulated kinase 1 blocks CKD-induced muscle wasting via inactivation of FoxO3a and Smad2/3. J Am Soc Nephrol. 2016;27(9):2797–808.
Kikuchi H, et al. Failure to sense energy depletion may be a novel therapeutic target in chronic kidney disease. Kidney Int. 2019;95(1):123–37.
Tang C, et al. P53 in kidney injury and repair: Mechanism and therapeutic potentials. Pharmacol Ther. 2018;195:5–12.
ÓhAinmhire E, et al. A conditionally immortalized Gli1-positive kidney mesenchymal cell line models myofibroblast transition. Am J Physiol Renal Physiol. 2018;316(1):F63–F75.
Randles MJ, et al. Genetic background is a key determinant of glomerular extracellular matrix composition and organization. J Am Soc Nephrol. 2015;26(12):3021–34.
Kato M, et al. Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: a novel mechanism related to diabetic kidney disease. J Am Soc Nephrol. 2006;17(12):3325–35.
Zhang W, et al. Platycodon grandiflorum saponins ameliorate cisplatin-induced acute nephrotoxicity through the NF-kappaB-mediated inflammation and PI3K/Akt/apoptosis signaling pathways. Nutrients. 2018;10(9):1328.
Zhang Aiqing, et al. Wang, miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J Cachexia Sarcopenia Muscle. 2018;9(4):755–70.
Wang Y, et al. Influence of erythropoietin on microvesicles derived from mesenchymal stem cells protecting renal function of chronic kidney disease. Stem Cell Res Ther. 2015;6(1):100.
Acknowledgements
The study was supported by grants from the National Natural Sciences Foundation of China (81870498, 81900633).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zhang, W., Yi, B., Yang, SK. et al. Extracellular vesicles carrying miRNAs in kidney diseases: a systemic review. Clin Exp Nephrol 24, 1103–1121 (2020). https://doi.org/10.1007/s10157-020-01947-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-020-01947-z