Abstract
Background
The relationship between serum corrected calcium (CCa) level and vessel calcification at dialysis initiation and survival has seldom been evaluated. Therefore, we evaluated the efficacy of CCa levels and the calcification score at the initiation of dialysis for predicting all-cause and cardiovascular (CV) mortality in patients with end-stage renal disease (ESRD).
Methods
The study group included 407 patients with ESRD, who started hemodialysis between January 2009 and December 2016 at the Red Cross Ishinomaki Hospital. The primary outcomes were the 1- and 3-year all-cause and CV mortality rate, with the association between CCa level and CVD-specific mortality evaluated using the Kaplan–Meier method and Cox proportional hazard regression analysis.
Results
Patients with a high initial CCa level were at higher risk for CVD-related, but not all-cause, mortality than patients with a low initial CCa level [hazard ratio (HR) 2.81; 95% confidence interval 1.05–7.55]. The HR for CVD-related mortality was also higher for patients with an Agatston vessel calcification score > 2000 (HR 13.9; 95% CI 1.63–118.2). Overall, the 3-year CVD-free rate was 88.2% (range 76.4–94.3%). Higher CCa level was associated with a higher Agatston score and cardiac valve calcification.
Conclusion
High serum CCa levels and an Agatston score > 2000 are independent risk factors of CVD mortality due to advanced vessel calcification.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality among patients on hemodialysis (HD) and, therefore, assessing CVD risk factors in these patients is clinically meaningful [1]. Several factors, including high-sensitivity C-reactive protein (hs-CRP) and serum magnesium, have been reported to be predictive markers of CV mortality in patients on HD [2, 3]. However, the relationship between serum CCa and the incidence rate of all-cause and CVC-specific mortality in this clinical population has not been evaluated. As an elevated CCa level increases the risk for vessel calcification in patients with end-stage renal disease (ESRD), serum CCa would be a risk factor for CVC-specific mortality. In ESRD, CCa levels can be lowered by an increase in serum levels of phosphate and intact parathormone (PTH), secondary to hyperparathyroidism (2HPT) [4], although such lowering of CCa levels is not observed in all the patients at dialysis initiation.
According to a survey of more than 30,000 incidental HD patients, conducted by the Japanese Society of Dialysis Therapy, the serum CCa level at the time of dialysis initiation is negatively correlated to all-cause mortality. Using a Cox hazard proportional analysis, adjusted for sex, age, etiology of ESRD, and estimated glomerular filtration rate (eGFR), a CCa level between 8 and 9 mg/dL at dialysis initiation is associated with the best survival prognosis. The hazard ratio (HR) of all-cause mortality for a serum CCa level ≤ 9 mg/dL is 1.58 (95% confidence interval [CI] 1.40–1.77 mg/dL) compared to 2.16 (95% CI 1.82–2.18) for a level ≤ 10.0 mg/dL. However, although a high serum CCa level has been recognized as an independent risk factor for all-cause mortality [5], its relationship to CVC-specific mortality was not investigated. A recent report on the serum CCa levels at dialysis initiation more completely showed that, in a Japanese cohort of 1524 incidental HD patients, a high level of CCa was associated with a higher incidence of all-cause mortality [6]. The authors suggested that patients with high CCa were introduced to HD at a higher eGFR because of advanced organ calcification. Again, however, the direct relationship between CCa level and CV mortality was not evaluated. Therefore, our aim in this study was to evaluate the relationship between CCa level at the time of dialysis initiation and all-cause and CV mortality using, not only the reported calcification score, but also the abdominal aorta calcification (AAC) score.
Patients and methods
Study population
We conducted a single-center, retrospective, observational study of the 437 patients with ESRD, > 20 years of age, who began incident HD between January 2009 and December 2016 at the Japanese Red Cross Ishinomaki Hospital. Of these patients, the clinical course of 407 patients was followed-up for over a period of at least 3 months, up to March 2017. The other 24 patients were lost to follow-up during the period of observation (Fig. 1). The mean duration of the observation period was 990.0 (range 496.5–1828.5) days. The cause of ESRD in this cohort included the acute kidney injury and rapid progressive glomerulonephritis as well as patients with normal progressive chronic kidney disease. The primary endpoints of this study were all-cause and CV mortality.
Definitions
All blood samples were collected once at the beginning of the first dialysis session. If the serum albumin level was < 4.0 g/dL, the serum Ca concentration was corrected by the serum albumin level as follows: corrected Ca (mg/dL) = measured Ca (mg/dL) + (4-albumin [g/dL]). Blood pressure (BP) was recorded for each patient before the first session of dialysis, following a 5-min rest period. Hypertension and hyperlipidemia were defined as per the Japanese guidelines [7, 8]. Height was obtained at the first dialysis session and body weight at the final dialysis session. The body mass index (BMI) was calculated by dividing the weight (kg) by the height (m2). We clinically examined patients for signs of fluid overload on the day before discharge, using chest radiography and ultrasound echocardiography. A positive history of CVD included the following: coronary artery disease (CAD) requiring percutaneous coronary intervention or coronary artery bypass graft surgery; aortic dissection; heart failure requiring hospitalization and in-patient treatment; and atrial fibrillation (AF). Data of comorbidities, including diabetes mellitus, hypertension, hyperlipidemia, CVD, and hemorrhagic stroke, were collected from patients’ electronic medical records. Our blood sample data were not affected by the electrolyte concentration of dialysate, which was the same for all the participants in our dialysis facility.
Calcification measurement
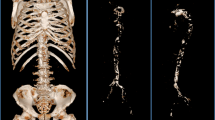
We measured the abdominal aorta calcification (AAC) using the Agatston score, which was used to quantify CAC [9]. We also used the calcification measurement using the ziostation2 software (Ziosoft Corp.). For AAC determination, the abdominal aorta was divided into intervals of 7 mm, from the celiac arterial bifurcation to the total iliac artery bifurcation, and the AAC was calculated using the computed tomography (CT) value and the calcification area. This score has previously been shown to positively correlate with coronary artery calcification and intima media thickness [10]. Furthermore, we repeated the Agatston score in 88 cases at 1 year post-dialysis initiation. Cardiac valve calcification was evaluated based on the presence or absence of a calcified aortic valve and mitral valve [11].
Laboratory and statistical analysis
The serum Ca level on the first dialysis session was available for all 407 patients forming out the study cohort. Using the corrected calcium levels, patients were divided into the following three groups: G1 < 8.0 mg/dL (7.1 ± 0.7 mg/dL); 8.0 ≤ G2 < 9.0 mg/dL (8.4 ± 0.2 mg/dL) and G3 ≥ 9.0 mg/dL (9.2 ± 0.7 mg/dL). Between-group variables were presented as the mean ± standard deviation or median with interquartile range (25th to 75th percentiles), as appropriate for the data type. Between-group differences were evaluated using Student’s t test for variables with a normal distribution and the Mann–Whitney U test for variables with a non-normal distribution. The Fisher exact test was used to evaluate differences between categorical values, and these results were expressed as an absolute (n) and percentage (%). The survival rate was assessed using the Kaplan–Meier analysis, with between-group differences evaluated using the log-rank test. The association between the serum CCa level and CVC-specific mortality was initially examined using the univariate Cox proportional hazards model. The independence of prognostic variables was examined using multivariate Cox proportional hazard models. We selected the variables with a p value < 0.10 in univariate analysis or reported as clinically relevant to CV mortality. The following variables were evaluated as predictor variables: age, sex, ESRD etiology, smoking history, serum albumin, blood urea nitrogen (BUN), eGFR, hemoglobin, CRP, corrected Ca, serum phosphate, intact PTH, white blood cell (WBC), total cholesterol, ferritin, the history of CVD, ejection fraction (EF), and medication used (ACE-i/ARBs, calcium channel blocker, Ca carbonate, phosphate binder, vitamin D receptor activator, loop diuretics), and the 3-group stratification of the Agatston score (0 < Agatston score ≤ 1000; 1000 < Agatston score ≤ 2000; and 2000 < Agatston score).
All statistical analyses were performed using EZR, which is a graphical user interface for R (R Foundation for Statistical Computing). p values were two-sided, with p < 0.05 used as the threshold for significance.
Results
Patients’ baseline characteristics
Baseline characteristics are reported in Table 1. The mean age of the study sample was 66.1 ± 13.1 years, with 268 male patients (65.3%) included. Three-hundred twenty-nine patients (80.8%) received their first dialysis session through an arteriovenous fistula. The mean BMI was 24.8 ± 4.9 kg/m2. Levels of anemia-related markers were as follows: hemoglobin (Hb), 8.7 ± 1.6 g/dL; transferrin saturation, 0.23 ± 0.15; and serum ferritin, 101.0 ng/mL (range 53.0–195.0 ng/mL). Renal-related markers were as follows: BUN level, 104.2 ± 34.4 mg/dL; serum creatinine level, 9.8 ± 3.1 mg/dL; and eGFR, 4.9 ± 1.9 mL/min/1.73 m2. Compared to G1, G3 had a significantly lower ALB level. Considering markers related to secondary hyperparathyroidism, intact PTH and serum phosphate levels were significantly lower in G3 than in G1. Considering the renal function markers in G3, eGFR was higher and the BUN level was lower than for patients in G1. Regarding cardiac function, left ventricular ejection fraction (LVEF), and brain natriuretic peptide (BNP) levels were similar among the three groups. There were no significant between-group differences with regard to medication use (with the exception of loop diuretic and phosphate binder) or ESRD etiology. The prevalence of CVD history was lower for G1 than for G2 and G3. Finally, considering the symptoms that directly caused dialysis initiation, the rate of acute heart failure was significantly higher in G3 that in the other two groups: G3 (10.7%) versus G2 (6.9%) versus G1 (2.8%), p = 0.03.
With regard to the causes of mortality, 20 patients died of an infectious disease, 27 of CVD (including 3 due to CAD, 24 due to HF), 9 of a hemorrhagic stroke, 11 of a malignancy, 2 of a gastrointestinal hemorrhage, and 27 of an unknown reason or accident.
Patient survival rate
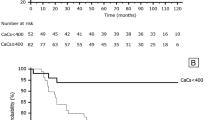
Kaplan–Meier curves revealed a non-significant tendency to a lower survival rate among patients in the G3 compared to the other groups. The 3-year survival rates in G3 were 74.4% (95% CI 62.0–83.7). The rate of CVC-specific mortality was higher in G3 than the middle (G2) or low (G1) groups (p < 0.01, Fig. 2). The 1- and 3-year CVD-free mortality rates in G3 were 95.8% (95% CI 87.5–98.6) and 88.2% (95% CI 76.4–94.3%), respectively (Table 2). In the Cox hazard survival models, CCa was found to be an independent risk factor of CVD-free mortality, after adjusting for several factors. After adjusting the models, the hazard ratio (HR) of CVD-free mortality was 2.81 (95% CI 1.05–7.55) and Agatston score > 2000 was 13.9 (range 1.63–118.2). In a secondary analysis that was performed including only patients > 65 years, the incidence of CV mortality was significantly higher in G3 than in G2 or G1 (Supplementary Figure 1). In the Cox hazard survival analysis, CCa and an Agatston score > 2000 were also identified as independent risk factors of CV mortality, using the same adjustment of the model, with a HR of 4.73 (95% CI 1.18–18.9) for CCa and 17.56 (95% CI 1.49–207.4) for the Agatston score > 2000 (Table 3). For the subsequent analysis, patients were sub-classified into two groups based on the presence or absence of a prior CAD history, with 346 patients classified in the CAD (−) group and 61 in the CAD (+) group. In the CAD (−) group, CVC-specific mortality was significantly higher in G1 than in either G2 and G3. In the CAD (+) group, no significant between-group difference was identified (Fig. 3). The 1- and 3- year CVC-specific mortality rate in G3 were 96.6% (95% CI 86.9–99.1) and 91.6% (95% CI 78.7–96.8), respectively (Table 4).
Calcification score
The incidence rate of mitral valve calcification was significantly greater in G3 (17.1%) than G1 (11.2%; p = 0.04), with a non-significant tendency to higher aortic valve calcification as well. The Agatston score was lower in G1 than either G2 or G3 (G1, 1000.6 [range 266.6–2277.8]; G2, 1628.2 [range 439.8–3356.9]; and G3, 1122.5 [range 202.5–2689.3]; p = 0.04); Fig. 4. The Agatston score at dialysis initiation and at 1 year after dialysis initiation was strongly and positively correlated (r = 0.875, p < 0.001; Fig. 5).
a Comparison of aortic and mitral valve calcification among the three groups using Fishers’ exact test. Significant difference was observed in mitral valve calcification (p = 0.04). Aortic valve calcification tended towards a significant difference (p = 0.06). b Abdominal aorta calcification, evaluated using the Agatston score using the Kruskal–Wallis test. The low CCa group had a lower grade of calcification than the other two groups (p = 0.04)
Discussion
This is the first observational study to have evaluated the relationship between CCa and CV mortality, including calcification scores, the Agatston score, at the time of dialysis initiation. We identified an elevated CCa level and Agatston score to be an independent risk factor of CV mortality, regardless of history of CAD.
Several causes of elevated CCa at the time of dialysis initiation are conceivable in our study cohort. First, the frequency of CVD history, such as CAD and HF hospitalization, were higher in G3 than in G1, such that the frequency of dialysis initiation due to acute heart failure, caused by excess fluid volume, was higher in G3. For this reason, compared to patients in G1, patient in G3 had a lower dialysis initiation frequency through their arteriovenous fistula, lower nutritional status (ALB) and higher eGFR at dialysis initiation. Furthermore, because of ESRD-related secondary hyperparathyroidism, serum phosphate and intact PTH levels were significantly lower in G3 than in G1. Second, the cardiac valve calcification and AAC score were significantly higher in G3 than G1. Taking into consideration that the Agatston score was significantly higher among patients with a history of CVD or hemorrhagic stroke, vessel calcification was severe among patients in G3, due to aging and a history of CVD [12]. Considering the age- and CVD-related calcification among patients in G3 at dialysis initiation, it is likely that a history of heart failure may have also contributed to high vascular calcification. Moreover, in these patients, the control of body fluid is often difficult due to the progression of ESRD and earlier dialysis initiation at a higher eGFR. As such, the decrease in serum Ca level is small due to uremia. However, it is controversial whether these factors directly influence the prognosis for survival. In our study (Table 1), the average eGFR was within 4.5–5.3 mL/min/1.73 m2 across all three groups and, therefore, eGFR level alone likely did not influence survival prognosis. In the Japanese study of 37 000 incidental HD patients, Yamagata et al. [13] reported a eGFR ≥ 6.0 mL/min/1.73 m2 to be an independent risk factor of all-cause mortality. The eGFR among patients in our study group did not reach this level. Although there were no significant between-group differences in LVEF, BNP, and cardio thoracic ratio in our study, the frequency of dialysis initiation caused by acute heart failure was significantly higher in G3 than in G2. Of the markers related to heart failure, the Agatston score was a more useful predictor of CV mortality than serum level parameters, such as BNP and eGFR.
In addition, we identified a significant between-group difference with regard to CVD-specific CV mortality, even among patients without a history of CAD. Specifically, coronary artery stenosis, with a narrowing ≥ 75 of the normal reference segment being clinically significant [14, 15], was higher in the CAD (−) patients in G3 than the CAD (−) patients in G1 or G2. Joki et al. reported a high prevalence rate of CAD of 62.5% (15 of 24 cases) among patients with ESRD prior to dialysis initiation [16]. If body fluid management becomes difficult due to the progression of uremia, heart failure develops, requiring early dialysis initiation [17]. In our study group, asymptomatic coronary artery stenosis was present in many patients, which likely contributed to the higher CVD-specific mortality rate.
We compared multiple research data at dialysis initiation in Japan from the viewpoint of renal anemia and 2HPT (Table 5). In our study, the level of intact PTH was lower compared to levels reported in other cohort studies conducted in Japan, despite of a lower frequency of using VDRA. We hypothesized that this lower level of intact PTH in our cohort likely reflects differences in local diet. Specifically, Ishinomaki City, where this study was performed, is a famous fishing port in Japan and, therefore, the consumption of fish as a staple is higher than in the other studies. Fish contains high levels of vitamin D, which is important in suppressing the level of intact PTH [18, 19]. As well, the level of Hb was lower in our study cohort than levels reported in other Japanese cohorts. Specifically, two previous studies have reported a Hb level < 8.0 g/dL to be a significant prognostic factor at dialysis initiation [20, 21]; the mean Hb level in our study cohort was above this cut-off value. Since 2007, Japanese nephrologists have been using long-active ESA (darbepoetin alfa, epoetin beta pegol) to treat patients with CKD, which has improved the management of renal anemia [22]. Studies having evaluated the relationship between the Hb level and CV mortality to date, however, have not included markers of vascular calcification in their analysis, a factor which is known to be strongly associated with CV events. In this study, we did not identify a significant difference in the frequency and types of ESA used. Furthermore, in our Cox hazard multivariate analysis, Hb level was not identified to be an independent risk factor of CV-related mortality.
The degree of calcification at the time of dialysis initiation will influence the future progression of calcification [24]. We monitored the Agatston score over 1 year after dialysis initiation in 88 cases, demonstrating a strong correlation between the score at the time of dialysis initiation and at 1 year post-initiation. The higher the calcification score, the greater the vascular calcification, which facilitated the development of CVD. From these results, we conclude that hypercalcification at the time of dialysis initiation is a strong risk factor for CVD events.
Agents that affect serum Ca levels may have influenced serum Ca levels [25]. As shown in Table 1, calcium carbonate, vitamin D receptor activator, and thiazide diuretics were not significantly different among the groups. Loop diuretics varied among the group; however, the influence of loop diuretics on serum Ca levels is weak [26], with Ca carbonate having the greatest influence on serum Ca level. However, Ca carbonate levels were not available for our study group.
The limitations of our study should be acknowledged. First, this was a cross-sectional, observational, single-center study. Second, ESRD etiology was not confirmed by biopsy in all cases, with some patients having only a clinical diagnosis. Third, we were unable to investigate drug doses, including Ca carbonate which is known to affect serum Ca levels. Fourth, we had no data regarding peripheral arterial disease, including CVD, or clinical frailty scores. Finally, blood samples for biochemical analysis were only obtained at the first dialysis session.
Conclusions
A high level of serum CCa was identified as an independent risk factor for CV mortality. A high serum CCa level was associated with advanced vessel calcification, with severe heart, and other organ, damage. Thus, the difficulty of controlling excess fluid in these patients leads to acute heart failure, prompting the need for early HD. Therefore, a high level of serum CCa, coupled with advanced vessel calcification, increases the risk of CVD-specific mortality among patients with ESRD.
References
Locatelli F, Pozzoni P, Tenori F, Vecchio LD. Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol Dial Transplant. 2003;18:viii2–9.
Antunovic T, Stefanovic A, Gligorovic BN, Milijkovic M, Radunovi D, Ivanisevic J, et al. Pro-oxidant-antioxidant balance, hsTul and hsCRP: mortality prediction in haemodialysis patients, two-year follow-up. Ren Fail. 2017;39:491–9.
Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85:174–81.
Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutics options. Clin J Am Soc Nephrol. 2011;6:913–21.
Hanafusa N, Nakai S, Iseki K, Tsubakihara Y. Japanese society for dialysis therapy renal data registry-a window through which we can view the details of Japanese dialysis population. Kidney Int Suppl. 2015;5:15–22.
Inaguma D, Koide S, Takahashi K, Hayashi H, Hasegawa M, Yuzawa Y. Relationship between serum calcification level at dialysis initiation and subsequent prognosis. Ren Replace Ther. 2017;3:2.
Obara T, Ubeda SR, Ohkubo T, Matsuura H, Ishimitsu T, Takata M, et al. Awareness of the Japanese Society of Hypertension Guidelines for the Management of Hypertension and their use in clinical practice: 2009 survey results. Hypertens Res. 2015;38:400–4.
Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, et al. Japan Atherosclerosis Society Committee for Epidemiology and Clinical Management of Atherosclerosis: statements. J Atheroscler Thromb. 2014;21:299–303.
Ichii M, Ishimura E, Shima H, Ohno Y, Ochi A, Nakatani S, et al. Quantitative analysis of abdominal aortic in CKD patients without dialysis therapy by use of the Agatston score. Kidney Blood Press Res. 2013;38:196–204.
Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, et al. Coronary artery calcification compared with carotid initima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic study of atherosclerosis (MESA). Arch Intern Med. 2008;168:1333–9.
Kim IY, Kim MJ, Lee DW, Lee SB, Shin MJ, Rhee H, et al. Cardiac valve calcification is associated with presence and severity of coronary artery disease in patients with pre-dialysis chronic kidney disease. Clin Exp Nephrol. 2015;19:1090–7.
Brancaccio D, Bellasi A, Cozzlino M, Galassi A, Gallieni M. Arterial accelerated aging in dialysis patients: the clinical impact of vascular calcification. Curr Vasc Pharmacol. 2009;7:374–80.
Yamagata K, Nakai S, Iseki K, Tsubakihara Y. Committee of Renal Data Registry of the Japanese Society for Dialysis Therapy. Late dialysis start did not affect long-term outcome in Japanese dialysis patients: long-term prognosis from Japanese society for dialysis therapy registry. Ther Apher Dial. 2012; 16:111–20.
Feuchtner GM, Mullers S, Grander W, Alber HF, Bartel T, Friedrich GJ, et al. Aortic valve calcification as quantified with multislice computed tomography predicts short-term clinical outcome in patients with asymptomatic aortic stenosis. J Heart Valve Dis. 2006;15:494–8.
Joki N, Hase H, Nakamura T, Yamaguchi T. Onset of coronary artery disease prior to initiation haemodialysis in patients with end-stage renal disease. Nephrol Dial Transplant. 1997;12:718 – 23.
JCS Joint Working Group. Guidelines for treatment of acute heart failure (JCS 2011). Cir J. 2013; 77:2157–201.
Harnett JP, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis, and risk factors. Kidney Int. 1995;47:884 – 90.
Nakamura K, Nashimoto M, Okuda Y, Ota T, Yamamoto M. Fish as a major source of vitamin D in the Japanese diet. Nutrition. 2002;18:415–6.
Holvik K, Madar AA, Meyer HE, Lofthus CM, Stene LC. Changes in the vitamin D endocrine system and bone turnover after oral vitamin D3 supplementation in healthy adults: results of a randomised trial. BMC Endocr Disord. 2012;12:7.
Kataoka H, Tsuchiya K, Naganuma T, Okazaki M, Komatsu M, Kimura T, et al. Relationship prognosis between anemia management at hemodialysis initiation and patient. Nephrology. 2015;20:14–21.
Watanabe Y, Akizawa T, Saito A, Gejyo F, Suzuki M, Nishizawa Y, et al. Effect of predialysis recombinanth human erythropoietin on early survival after hemodialysis initiation in patients with chronic kidney disease: Co-JET study. Ther Apher Dial. 2016;20:598–607.
Kato H, Nangaku M, Hirakata H, Wada T, Hayashi T, Sato H, et al. Rationale and design of observational clinical research in chronic kidney disease patients with renal anemia: renal prognosis in patients with hyporesponsive anemia to erythropoiesis-stimulating agents, darbepoetiN alfa (BRIGHTEN Trial). Clin Exp Nephrol. 2017. https://doi.org/10.1007/s10157-017-1427-4.
Asakawa T, Joki N, Tanaka Y, Hayashi T, Hase H, Komatsu Y, et al. Association between the hemoglobin level and cardiothoracic ratio in patients on incident dialysis. Cardiorenal Med. 2014;4:189–200.
Lee MJ, Shin DH, Kim SJ, Oh HJ, Yoo DE, Ko KI, et al. Progression of aortic arch calcification over 1 year is an independent predictor of mortality in incident peritoneal dialysis patients. PLoS One. 2012;7:e48793.
Ruppe MD. Medications that affect calcium. Endocr Pract. 2011;17:26–30.
Griff M, Bushinsky DA. Diuretics and disorders of calcium homeostasis. Semin Nephrol. 2011; 31:535–41.
Acknowledgements
We thank the staff of the Ishinomaki Red Cross Hospital for their help with conducting this study and give special thanks to Kazuya Takahashi, Radiation Technology Division.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB Approval number: 14–20) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sato, H., Nagasawa, T., Saito, A. et al. Risk of cardiovascular mortality predicted by the serum calcium level and calcification score at the initiation of dialysis. Clin Exp Nephrol 22, 957–966 (2018). https://doi.org/10.1007/s10157-017-1527-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1527-1