Abstract
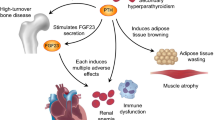
Secondary hyperparathyroidism (SHPT) is a common complication in chronic kidney disease. Currently, various treatment options are available, including vitamin D receptor activators, cinacalcet hydrochloride, and parathyroidectomy. These treatment options have contributed to the successful control of SHPT, and recent clinical studies have provided evidence suggesting that effective treatment of SHPT leads to improved survival. Although bone disease is the most widely recognized consequence of SHPT and remains a major target for treatment of SHPT, there is increasing evidence that parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23), both of which are markedly elevated in SHPT, have multiple adverse effects on extraskeletal tissues. These actions may lead to the pathological development of left ventricular hypertrophy, renal anemia, immune dysfunction, inflammation, wasting, muscle atrophy, and urate accumulation. Given that treatment of SHPT leads to decreases in both PTH and FGF23, these data provide an additional rationale for treating SHPT. However, definitive evidence is still lacking, and future research should focus on whether treatment of SHPT prevents the adverse effects of PTH and FGF23.


Similar content being viewed by others
References
Komaba H, Kakuta T, Fukagawa M. Diseases of the parathyroid gland in chronic kidney disease. Clin Exp Nephrol. 2011;15:797–809.
Slatopolsky E, Delmez JA. Pathogenesis of secondary hyperparathyroidism. Nephrol Dial Transplant. 1996;11(Suppl 3):130–5.
Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8.
Komaba H, Fukagawa M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 2010;77:292–8.
Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82:737–47.
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–8.
Komaba H, Goto S, Fujii H, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232–8.
Galitzer H, Ben-Dov IZ, Silver J, et al. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–8.
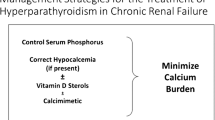
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–130.
Guideline Working Group, Japanese Society for Dialysis Therapy. Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial. 2008;12:514–25.
Palmer S, McGregor D, Macaskill P, et al. Meta-analysis. vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147:840–53.
Okuno S, Ishimura E, Kitatani K, et al. Relationship between parathyroid gland size and responsiveness to maxacalcitol therapy in patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2003;18:2613–21.
Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–25.
Tominaga Y, Numano M, Tanaka Y, et al. Surgical treatment of renal hyperparathyroidism. Semin Surg Oncol. 1997;13:87–96.
Hruska KA, Teitelbaum SL. Renal osteodystrophy. N Engl J Med. 1995;333:166–74.
Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–18.
Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008;52:519–30.
Taniguchi M, Fukagawa M, Fujii N, et al. Serum phosphate and calcium should be primarily and consistently controlled in prevalent hemodialysis patients. Ther Apher Dial. 2013;17:221–8.
Portale AA, Booth BE, Halloran BP, et al. Effect of dietary phosphorus on circulating concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderate renal insufficiency. J Clin Invest. 1984;73:1580–9.
Ketteler M, Rix M, Fan S, et al. Efficacy and tolerability of sevelamer carbonate in hyperphosphatemic patients who have chronic kidney disease and are not on dialysis. Clin J Am Soc Nephrol. 2008;3:1125–30.
Slatopolsky E, Finch J, Denda M, et al. Phosphorus restriction prevents parathyroid gland growth. High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest. 1996;97:2534–40.
Almaden Y, Canalejo A, Hernandez A, et al. Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res. 1996;11:970–6.
Slatopolsky E, Weerts C, Thielan J, et al. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest. 1984;74:2136–43.
Reynolds JL, Joannides AJ, Skepper JN, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–67.
Llach F, Yudd M. Paricalcitol in dialysis patients wtih calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis. 2001;38(Suppl 5):S45–50.
Akizawa T, Suzuki M, Akiba T, et al. Long-term effect of 1,25-dihydroxy-22-oxavitamin D3 on secondary hyperparathyroidism in haemodialysis patients. One-year administration study. Nephrol Dial Transplant. 2002;17(Suppl 10):28–36.
Fukuda N, Tanaka H, Tominaga Y, et al. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993;92:1436–43.
Kifor O, Moore FD Jr, Wang P, et al. Reduced immunostaining for the extracellular Ca2þ-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81:1598–606.
Messa P, Macário F, Yaqoob M, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3:36–45.
Komaba H, Nakanishi S, Fujimori A, et al. Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010;5:2305–14.
St Peter WL, Li Q, Liu J, et al. Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol. 2009;4:354–60.
Vervloet M, Bencova V, Malberti F, et al. “Real-World” use of cinacalcet for managing SHPT in different European countries: analysis of data from the ECHO observational study. Clin Nephrol. 2010;74:198–208.
Tominaga Y, Kakuta T, Yasunaga C, et al. Evaluation of parathyroidectomy for secondary and tertiary hyperparathyroidism by the Parathyroid Surgeons’ Society of Japan. Ther Apher Dial. 2016;20:6–11.
Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10:98–109.
Martin KJ, Pickthorn K, Huang S, et al. AMG 416 (velcalcetide) is a novel peptide for the treatment of secondary hyperparathyroidism in a single-dose study in hemodialysis patients. Kidney Int. 2014;85:191–7.
Fukagawa M, Yokoyama K, Shigematsu T, et al. A phase 3, multicentre, randomised, double-blind, placebocontrolled, parallel-group study to evaluate the efficacy and safety of etelcalcetide (ONO-5163/AMG 416), a novel intravenous calcimimetic, for secondary hyperparathyroidism in Japanese haemodialysis patients. Nephrol Dial Transplant. (in press).
Kitaoka M, Fukagawa M, Ogata E, et al. Reduction of functioning parathyroid cell mass by ethanol injection in chronic dialysis patients. Kidney Int. 1994;46:1110–7.
Shiizaki K, Hatamura I, Negi S, et al. Percutaneous maxacalcitol injection therapy regresses hyperplasia of parathyroid and induces apoptosis in uremia. Kidney Int. 2003;64:992–1003.
Yajima A, Tanaka K, Tominaga Y, et al. Early changes of bone histology and circulating markers of bone turnover after parathyroidectomy in hemodialysis patients with severe hyperparathyroidism. Clin Nephrol. 2001;56:27–34.
Tominaga Y, Katayama A, Sato T, et al. Re-operation is frequently required when parathyroid glands remain after initial parathyroidectomy for advanced secondary hyperparathyroidism in uraemic patients. Nephrol Dial Transplant. 2003;18(Suppl 3):iii65–70.
Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70:1358–66.
Abdelhadi M, Nordenström J. Bone mineral recovery after parathyroidectomy in patients with primary and renal hyperparathyroidism. J Clin Endocrinol Metab. 1998;83:3845–51.
Rudser KD, de Boer IH, Dooley A, et al. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol. 2007;18:2401–7.
Behets GJ, Spasovski G, Sterling LR, et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;87:846–56.
Moe SM, Abdalla S, Chertow GM, et al. Effects of cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE trial. J Am Soc Nephrol. 2015;26:1466–75.
Salusky IB, Kuizon BD, Belin TR, et al. Intermittent calcitriol therapy in secondary hyperparathyroidism: a comparison between oral and intraperitoneal administration. Kidney Int. 1998;54:907–14.
Komaba H, Taniguchi M, Wada A, et al. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;88:350–9.
Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among US dialysis patients. Kidney Int. 2004;66:2010–6.
Costa-Hong V, Jorgetti V, Gowdak LH, et al. Parathyroidectomy reduces cardiovascular events and mortality in renal hyperparathyroidism. Surgery. 2007;142:699–703.
Ivarsson KM, Akaberi S, Isaksson E, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant. 2015;30:2027–33.
EVOLVE Trial Investigators. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–94.
Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, Pisoni RL. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014;85:166–73.
Komaba H, Fukagawa M. Phosphate-a poison for humans? Kidney Int. 2016;90:753–63.
Streja E, Lau WL, Goldstein L, et al. Hyperphosphatemia is a combined function of high serum PTH and high dietary protein intake in dialysis patients. Kidney Int Suppl. 2013;3:462–8.
Cuppari L, de Carvalho AB, Avesani CM, et al. Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol. 2004;15:2933–9.
Chou FF, Lee CH, Chen JB. General weakness as an indication for parathyroid surgery in patients with secondary hyperparathyroidism. Arch Surg. 1999;134:1108–11.
Kir S, Komaba H, Garcia AP, et al. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab. 2016;23:315–23.
Kir S, White JP, Kleiner S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–4.
Brancaccio D, Cozzolino M, Gallieni M. Hyperparathyroidism and anemia in uremic subjects: a combined therapeutic approach. J Am Soc Nephrol. 2004;15(Suppl 1):S21–4.
Trunzo JA, McHenry CR, Schulak JA, et al. Effect of parathyroidectomy on anemia and erythropoietin dosing in end-stage renal disease patients with hyperparathyroidism. Surgery. 2008;144:915–9.
Tanaka M, Yoshida K, Fukuma S, et al. Effects of secondary hyperparathyroidism treatment on improvement in anemia: results from the MBD-5D study. PLoS One. 2016;11:e0164865.
Geara AS, Castellanos MR, Bassil C, et al. Effects of parathyroid hormone on immune function. Clin Dev Immunol. 2010;2010:418695.
Tzanno-Martins C, Futata E, Jorgetti V, et al. Restoration of impaired T-cell proliferation after parathyroidectomy in hemodialysis patients. Nephron. 2000;84:224–7.
Schlüter KD, Piper HM. Cardiovascular actions of parathyroid hormone and parathyroid hormone-related peptide. Cardiovasc Res. 1998;37:34–41.
Custódio MR, Koike MK, Neves KR, et al. Parathyroid hormone and phosphorus overload in uremia: impact on cardiovascular system. Nephrol Dial Transplant. 2012;27:1437–45.
Drüeke T, Fauchet M, Fleury J, et al. Effect of parathyroidectomy on left-ventricular function in haemodialysis patients. Lancet. 1980;1:112–4.
Sugimoto R, Watanabe H, Ikegami K, et al. Down-regulation of ABCG2, a urate exporter, by parathyroid hormone enhances urate accumulation in secondary hyperparathyroidism. Kidney Int. (in press).
Latif W, Karaboyas A, Tong L, et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol. 2011;6:2470–7.
Odden MC, Amadu AR, Smit E, et al. Uric acid levels, kidney function, and cardiovascular mortality in US adults: National Health and Nutrition Examination Survey (NHANES) 1988–1994 and 1999–2002. Am J Kidney Dis. 2014;64:550–7.
Komaba H, Fukagawa M. The role of FGF23 in CKD—with or without Klotho. Nat Rev Nephrol. 2012;8:484–90.
Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408.
Coe LM, Madathil SV, Casu C, et al. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem. 2014;289:9795–810.
Rossaint J, Oehmichen J, Van Aken H, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126:962–74.
Singh S, Grabner A, Yanucil C, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90:985–96.
Lavi-Moshayoff V, Wasserman G, Meir T, et al. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–9.
Acknowledgements
This supplement is supported by the Grants from the Japanese Society for Kidney Bone Disease (JSKBD) and from the Research Meeting on Kidney and Metabolic Bone Disease. The authors thank the following investigators who participated in the historical cohort study of maintenance hemodialysis patients: Dr. Miho Hida, Dr. Takao Suga, Dr. Reika Tanaka, Dr. Kayoko Watanabe, Dr. Nobuyoshi Takagi, Dr. Hiroshi Kida, Dr. Mitsumine Fukui, Dr. Tateki Kitaoka, Dr. Tetsuo Shirai, Dr. Mikako Nagaoka, Dr. Tsuneyoshi Oh, Dr. Eiji Nakano, Dr. Takayuki Hashiguchi, Dr. Hirofumi Ishii, Dr. Koichi Shimizu, Dr. Yasuji Sugano, Dr. Toru Furuya, Dr. Naoto Ishida, Dr. Hiroyuki Ogura, Dr. Hiroaki Nakada, Dr. Miho Enomoto, and Dr. Tetsuya Kashiwagi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.K. has received honoraria, consulting fees, and/or Grant/research support from Bayer Yakuhin, Chugai Pharmaceutical, and Kyowa Hakko Kirin. T.K. has received honoraria from Chugai Pharmaceutical and Kyowa Hakko Kirin. M.F. has received honoraria, consulting fees, and/or Grant/research support from Bayer Yakuhin, Kyowa Hakko Kirin, and Torii Pharmaceutical.
About this article
Cite this article
Komaba, H., Kakuta, T. & Fukagawa, M. Management of secondary hyperparathyroidism: how and why?. Clin Exp Nephrol 21 (Suppl 1), 37–45 (2017). https://doi.org/10.1007/s10157-016-1369-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1369-2