Abstract
Background
Conversion from calcineurin inhibitor (CNI) to mTOR inhibitors may reduce and even halt the progression of chronic allograft dysfunction (CAD) which is the most important cause of renal allograft loss. We aimed to investigate the effects of conversion from CNI to everolimus on parameters of fibrosis, inflammation, glomerulotubular damage and vascular functions in renal transplant recipients.
Methods
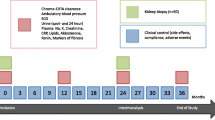
Fifteen stable renal transplant recipients who were under CNI treatment (male/female 13/2, mean age 41 ± 10 years) were enrolled and switched to everolimus. Serum and urinary transforming growth factor-β (TGF-β), urinary neutrophil gelatinase-associated lipocalin (NGAL) and monocyte chemoattractant protein-1 (MCP-1) were measured as markers of fibrosis, tubular damage and inflammation. As parameters of vascular functions, pulse wave velocity (PWV), augmentation index (AIx), serum asymmetric dimethyl-arginine and fibroblast growth factor-23 (FGF-23) were measured. All these measurements were repeated at the 3rd month of conversion.
Results
Estimated GFR (52 ± 7–57 ± 11 ml/min/l.73 m2, p = 0.02) (was increased after conversion to everolimus. However, serum uric acid levels were significantly decreased (6.21 ± 1.21–5.50 ± 1.39 mg/dL, p = 0.01). Serum TGF-β levels (8727 ± 2897–1943 ± 365 pg/mL, p = 0.03) and urinary NGAL levels (26 ± 10–12 ± 2 ng/mg creatinine, p = 0.05) were significantly decreased. However, urinary MCP-1, FGF-23, PWV and AIx did not change. Urinary TGF-β was associated with urinary NGAL (r = 0.62, p = 0.01), urinary MCP-1 (r = 0.68, p = 0.005) and proteinuria (r = 0.50, p = 0.05).
Conclusion
Conversion from CNI to everolimus resulted in significant decreases of serum TGF-β and urinary NGAL which may represent less fibrosis and tubular damage. Association of urinary TGF-β with NGAL and MCP-1 suggests that tubular damage, fibrosis and inflammation may act together for progression of CAD.



Similar content being viewed by others
References
Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605–12.
Calne RY, Rolles K, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Henderson RG, Aziz S, Lewis P. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–6.
Paul LC. Chronic allograft nephropathy: an update. Kidney Int. 1999;56(3):783–93.
Massy ZA, Guijarro C, Wiederkehr MR, Ma JZ, Kasiske BL. Chronic renal allograft rejection: Immunologic and nonimmunologic risk factors. Kidney Int. 1996;49:518–24.
Joannidès R, Monteil C, de Ligny BH, Westeel PF, Iacob M, Thervet E, Barbier S, Bellien J, Lebranchu Y, Seguin SG, Thuillez C, Godin M, Etienne I. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transpl. 2011;11(11):2414–22.
Seckinger J, Sommerer C, Hinkel UP, Hoffmann O, Zeier M, Schwenger V. Switch of immunosuppression from cyclosporine A to everolimus: impact on pulse wave velocity in stable de-novo renal allograft recipients. J Hypertens. 2008;26(11):2213–9.
Yong K, Nguyen HD, Hii L, Chan DT, Boudville N, Messineo A, Lim EM, Dogra GK, Lim WH. Association of a change in immunosuppressive regimen with hemodynamic and inflammatory markers of cardiovascular disease after kidney transplantation. Am J Hypertens. 2013.
Flechner SM, Kurian SM, Solez K, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. Am J Transpl. 2004;4:1776–85.
Bumbea V, Kamar N, Ribes D, Esposito L, Modesto A, Guitard J, Nasou G, Durand D, Rostaing L. Long-term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol Dial Transpl. 2005;20(11):2517–23.
Stallone G, Di Paolo S, Schena A, Infante B, Grandiliano G, Battaglia M, Gesualdo L, Scehna FP. Early withdrawal of cyclosporine improves 1 year kidney graft structure and function in sirolimus treated patients. Transplantation. 2003;75:998–1003.
Ruiz JC, Campistol JM, Grinyo JM, Mota A, Prats D, Gutierrez JA, Henriques AC, Pinto JR, Garcia J, Morales JM, Gomez JM, Arias M. Early cyclosporine A withdrawal in kidney transplant recipients receiving sirolimus prevents progression of chronic pathologic allograft lesions. Transplantation. 2004;78:1312–8.
Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–7.
Laurent S, Cockcroft J, Van BL, Boutouyrie P, Giannattasio C, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
Weber T, Ammer M, Rammer M, Adji A, O’rourke MF, et al. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens. 2009;27:1624–30.
Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326–33.
Klintmalm G, Bohman SO, Sundelin B, Wilczek H. Interstitial fibrosis in renal allografts after 12 to 46 months of cyclosporin treatment: beneficial effect of low doses in early post-transplantation period. Lancet. 1984;2:950–4.
Farnsworth A, Hall BM, Duggin GG, Horvath JS, Tiller DJ. Interstitial fibrosis in renal allografts in patients treated with cyclosporin. Lancet. 1984;2:1470–1.
Starzl TE, Fung J, Jordan M, Shapiro R, Tzakis A, McCauley J, Johnston J, Iwaki Y, Jain A, Alessiani M. Kidney transplantation under FK 506. JAMA. 1990;264:63–7.
Randhawa PS, Shapiro R, Jordan ML, Starzl TE, Demetris AJ. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506. Clinical significance and comparison with cyclosporine. Am J SurgPathol. 1993;17:60–68.
Lassila M. Interaction of cyclosporine A and the renin angiotensin system; new perspectives. Curr Drug Metab. 2002;3:61–71.
Khanna A, Plummer M, Bromberek C, Bresnahan B, Hariharan S. Expression of TGF-β and fibrogenic genes in transplant recipients with tacrolimus and cyclosporine nephrotoxicity. Kidney Int. 2002;62:2257–63.
Vieira JM Jr, Noronha IL, Malheiros DM, Burdmann EA. Cyclosporine-induced interstitial fibrosis and arteriolar TGF-β expression with preserved renal blood flow. Transplantation. 1999;68:1746–53.
Feldman G, Kiely B, Martin N, Ryan G, McMorrow T, Ryan MP. Role for TGF-β in cyclosporine-induced modulation of renal epithelial barrier function. J Am Soc Nephrol. 2007;18:1662–71.
Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–92.
Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-β pathway. Kidney Int. 2006;70:1914–9.
Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12.
Feldman G, Kiely B, Martin N, Ryan G, McMorrow T, Ryan MP. Role for TGF-β in cyclosporine-induced modulation of renal epithelial barrier function. J Am Soc Nephrol. 2007;18:1662–71.
Goc A, Choudhary M, Byzova TV, Somanath PR. TGFβ- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin [mTOR]. J Cell Physiol. 2011;226(11):3004–13.
Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 2006;69(11):2029–36.
Kurdián M, Herrero-Fresneda I, Lloberas N, Gimenez-Bonafe P, Coria V, Grande MT, Boggia J, Malacrida L, Torras J, Arévalo MA, González-Martínez F, López-Novoa JM, Grinyó J, Noboa O. Delayed mTOR inhibition with low dose of everolimus reduces TGFβ expression, attenuates proteinuria and renal damage in the renal mass reduction model. PLoS One. 2012;7(3):e32516.
Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin [NGAL] as a marker of kidney damage. Am J Kidney Dis. 2008;52(3):595–605.
Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Chapman JR. Delta analysis of posttransplantation tubulointerstitial damage. Transplantation. 2004;78(3):434–41.
Wasilewska A, Zoch-Zwierz W, Taranta-Janusz K, Michaluk-Skutnik J. Neutrophil gelatinase-associated lipocalin [NGAL]: a new marker of cyclosporine nephrotoxicity? Pediatr Nephrol. 2010;25(5):889–97.
Dubiński B, Boratyńska M, Kopeć W, Szyber P, Patrzałek D, Klinger M. Activated cells in urine and monocyte chemotactic peptide-1 [MCP-1]–sensitive rejection markers in renal graft recipients. Transpl Immunol. 2008;18(3):203–7.
Asai T, Nakatani T, Yamanaka S, Tamada S, Kishimoto T, Tashiro K, Nakao T, Okamura M, Kim S, Iwao H, Miura K. Magnesium supplementation prevents experimental chronic cyclosporine a nephrotoxicity via renin-angiotensin system independent mechanism. Transplantation. 2002;74(6):784–91.
Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17(11):2974–84.
Regateiro FS, Howie D, Cobbold SP, Waldmann H. TGF-β in transplantation tolerance. Curr Opin Immunol. 2011;23(5):660–9.
Budde K, Lehner F, Sommerer C, Arns W, Reinke P, Eisenberger U, Wüthrich RP, Scheidl S, May C, Paulus EM, Mühlfeld A, Wolters HH, Pressmar K, Stahl R, Witzke O, ZEUS Study Investigators. Conversion from cyclosporine to everolimus at 4.5 months posttransplant: 3-year results from the randomized ZEUS study. Am J Transpl. 2012;12(6):1528–40.
Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF. Reduced exposure to calcineurin inhibitors in renal transplantation. ELITE Symph Study N Engl J Med. 2007;357(25):2562–75.
Gill JS, Abichandani R, Kausz AT, Pereira BJ. Mortality after kidney transplant failure: The impact of non-immunologic factors. Kidney Int. 2002;62:1875–83.
Gómez-Marcos MA, Recio-Rodríguez JI, Patino-Alonso MC, Agudo-Conde C, Rodríguez-Sánchez E, Gómez-Sánchez L, Gómez-Sánchez M, García-Ortiz L. Relationship between uric acid and vascular structure and function in hypertensive patients and sex-related differences. Am J Hypertens. 2013;26(5):599–607.
Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, Kramer S, Fischer W, Gschaidmeier H, Pietruck F, ZEUS Study Investigators. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet. 2011;377(9768):837–47.
Perico N, Codreanu I, Caruso M, Remuzzi G. Hyperuricemia in kidney transplantation. Contrib Nephrol. 2005;147:124–31.
Panwalkar A, Verstovsek S, Giles FJ. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer. 2004;100:657–66.
Acknowledgements
This study was presented in American Transplant Congress in 18–22 May 2013. This study has been supported by Istanbul University Research Foundation with grant number 2012/827-1068.
Conflict of interest
All the authors have declared no competing interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Alpay, N., Ozkok, A., Caliskan, Y. et al. Influence of conversion from calcineurin inhibitors to everolimus on fibrosis, inflammation, tubular damage and vascular function in renal transplant patients. Clin Exp Nephrol 18, 961–967 (2014). https://doi.org/10.1007/s10157-014-0939-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-014-0939-4