Abstract
Background
Darbepoetin alfa (DA) is an attractive alternative to recombinant human erythropoietin (rHuEPO) in managing renal anemia. Since DA has not been approved by the appropriate Japanese drug regulatory agencies for the indication of renal anemia in children in Japan, we have conducted a multicenter prospective study to determine the efficacy and safety of DA in Japanese children undergoing peritoneal dialysis (PD).
Methods
Pediatric patients subcutaneously receiving rHuEPO were switched to DA treatment for a period of 28 weeks. The conversion to the initial dose of DA was calculated as 1 μg DA for 200 IU rHuEPO, and DA was administered intravenously once every 2 weeks. The target hemoglobin (Hb) concentration was defined as 11.0 to ≤13.0 g/dL. In some patients, the dose of DA was adjusted appropriately to achieve this target level, and/or the dosing frequency changed to once every 4 weeks.
Results
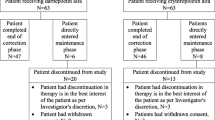
In the 25 patients switched from rHuEPO to DA the mean Hb concentration increased from 9.9 ± 1.0 to 11.1 ± 1.0 g/dL at 8 weeks following commencement of the DA treatment. The target Hb concentration was achieved in 88 % of these patients, and 60 % maintained this target value on completion of the study. The dosing frequency was extended to once every 4 weeks in 60 % of patients. Twenty-four adverse events were noted in 11 of 25 patients (44 %); however, there was no causality between DA and adverse events.
Conclusions
The results of this study suggest that intravenous administration of DA once every 2 or 4 weeks is an effective and safe treatment for renal anemia in Japanese children undergoing PD.







Similar content being viewed by others
References
Koshy SM, Geary DF. Anemia in children with chronic kidney disease. Pediatr Nephrol. 2008;23:209–19.
Gary Lerner, Kale AS, Warady BA, Jabs K, Bunchman TE, Heatherington A. Pharmacokinetics of darbepoetin alfa in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2002;17:933–7.
Brunkhorst R, Bommer J, Braum J, Hagg-Weber M, Gill C, Wagner J, et al. Darbepoetin alfa effectively maintains hemoglobin concentrations at extended dose intervals relative to intravenous or subcutaneous recombinant human erythropoietin in dialysis patients. Nephrol Dial Transplant. 2004;19:1224–30.
Jadoul M, Vanrenterghem Y, Foret M, Walker R, Gray SJ. Darbepoetin alfa administered once monthly maintains hemoglobin levels in stable dialysis patients. Nephrol Dial Transplant. 2004;19:898–903.
Hiramatsu M, Kubota M, Iwasaki M, Akizawa T, Kosikawa, and the KRN321 A09 Study Group. Darbepoetin alfa (KRN321) administered intravenously once monthly maintain hemoglobin levels in peritoneal dialysis patients. Ther Apher Dial. 2008;12:19–27.
Kubota M, Hiramatsu M, Yamakawa M, Fukuhara S, Morita S, Iwasaki M, et al. Darbepoetin alfa (KRN321) is safe and effective when administered subcutaneously once every 2 or 4 weeks to patients on peritoneal dialysis in Japan. Clin Exp Nephrol. 2011;15:884–92.
De Palo T, Giordano M, Palumbo F, Bellantuono R, Messina G, Colella V, et al. Clinical experience with darbepoetin alfa (NESP) in children undergoing hemodialysis. Pediatr Nephrol. 2004;19:337–40.
Geary DF, Keating LE, Vigneux A, Stephens D, Hebert D, Harvey EA. Darbepoetin alfa (AranespTM) in children with chronic renal failure. Kidney Int. 2005;68:1759–65.
Warady BA, Arar MY, Lerner G, Nakanishi AM, Stehman-Breen C. Darbepoetin alfa for the treatment of anemia in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2006;21:1144–52.
Andre JL, Deschenes G, Boudailliez B, Broux F, Fischbach M, Gagnadoux M-F, et al. Darbepoetin, effective treatment of anaemia in paediatric patients with chronic renal failure. Pediatr Nephrol. 2007;22:708–14.
Japanese Society for Dialysis Therapy. 2008 Japanese Society for Dialysis Therapy guideline for renal anemia in chronic kidney disease. J Jpn Soc Dial Ther 2008; 41:661–716.
Japanese Society for Dialysis Therapy. An overview of dialysis treatment in Japan (as of Dec. 31, 2008). J Jpn Soc Dial Ther 2010; 43:1–35.
Cummings EA, Reid GJ, Finley GA, McGrath PJ, Ritchie JA. Prevalence and source of pain in pediatric inpatients. Pain. 1996;68:25–31.
Schmitt CP, Nau B, Brummer C, Rosenkranz J, Schaefer F. Increase injection pain with darbepoetin-α compared to epoetin-β in paediatric dialysis patients. Nephrol Dial Transplant. 2006;21:3520–4.
Macdougal IC, Gray SJ, Elston O, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392–5.
Hiramatsu M, Kubota M, Yamamoto H. Limitation of rHuEPO treatment for target Hb in peritoneal dialysis patients (in Japanese). Kidney Dial. 2007;63:915–22.
North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). 2004 Annual Report. NAPRTCS Administrative Office, Boston, 2004.
Rijk Y, Raaijmakers R, Kar N, Schroder C. Intraperitoneal treatment with darbepoetin for children on peritoneal dialysis. Pediatr Nephrol. 2007;22:436–40.
Durkan AM, Keating LE, Vigneux A, Geary DF. The use of darbepoetin in infants with chronic renal impairment. Pediatr Nephrol. 2006;21:694–7.
Acknowledgments
The authors gratefully acknowledge Drs. Tomoyuki Sakai and Kenji Ishikura (Department of Nephrology, Tokyo Metropolitan Children’s Medical Center, Tokyo, Japan) and Yasushi Tsutumi (Department of Pediatrics, Kyushu University) for advice during this study. This study was supported by a grant from the Japan Dialysis Outcome Group (Grant #07009).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hattori, M., Matsunaga, A., Akioka, Y. et al. Darbepoetin alfa for the treatment of anemia in children undergoing peritoneal dialysis: a multicenter prospective study in Japan. Clin Exp Nephrol 17, 582–588 (2013). https://doi.org/10.1007/s10157-012-0714-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-012-0714-3