Abstract
Background
In response to the pandemic 2009 A/H1N1 virus, monovalent MF59-adjuvanted vaccines were prepared. Recently, single 3.75-μg doses of MF59-adjuvanted vaccines have shown good immunogenicity in young adults. However, the immunogenicity of these vaccines has not been evaluated in dialysis patients.
Methods
Dialysis patients received a single 3.75-μg dose of MF59-adjuvanted vaccine by intramuscular injection. For immunogenicity assays, serum samples were obtained before vaccination and 28 days after vaccination. All sera were tested by hemagglutination inhibition assays.
Results
Overall, 48 hemodialysis (HD) patients and 34 peritoneal dialysis (PD) patients were included in immunogenicity analysis. In HD patients, geometric mean titers (GMTs) were significantly increased compared with baseline GMTs in both young (aged 18–60 years) and elderly (aged ≥60 years) patients (51.2 ± 51.4 vs. 14.1 ± 20.7 in young patients, P = 0.012; 37.9 ± 73.9 vs. 6.8 ± 8.0 in elderly patients, P = 0.018, respectively). The rates of seroprotection and seroconversion were 27.6 and 17.2 % in young patients and 31.6 and 26.3 % in elderly patients, respectively. Among PD patients, GMTs were increased only in young patients (39.8 ± 51.4 vs. 6.8 ± 5.0, P = 0.001). The rates of seroprotection and seroconversion were 36.0 and 36.0 % in young patients and 11.1 and 0.0 % in elderly patients, respectively.
Conclusion
A single 3.75-μg dose of MF59-adjuvanted vaccine was suboptimal to elicit protective antibody response in dialysis patients. Antibody responses against vaccine were compromised especially in elderly PD patients. Trials of different vaccination protocols such as a two-dose schedule or a higher hemagglutinin antigen dose of MF59-adjuvanted vaccine are necessary for improving antibody response in dialysis patients.
Similar content being viewed by others
Introduction
In April 2009, emergence of a novel swine origin H1N1 influenza A virus was reported in Mexico and the United States. The rapid global spread of influenza A/H1N1 resulted in the World Health Organization (WHO) declaring a pandemic on June 11th 2009 [1]. The pandemic A/H1N1 virus generally caused mild and self-limiting illness, which was less pathogenic than had been anticipated. The WHO classified the influenza A/H1N1 2009 pandemic as being of moderate severity [2], but the majority of deaths occurred in people with underlying medical conditions such as end-stage renal disease. From June to September 2009, Marcelli et al. [3] reported 306 cases of influenza A/H1N1 in dialysis units. Among them, 34 % were hospitalized and 5 % died.
Vaccine is one of the most effective methods to reduce morbidity and mortality. In the early stage of pandemic, the European Medicine Agency recommended a two-dose schedule based on the unsatisfactory immune response against the avian influenza vaccine [4, 5]. However, several clinical trials have found that even a single dose of pandemic vaccine is sufficient to elicit a protective antibody response in healthy adults [6–9]. As most countries adopt a single-dose schedule, a much larger population was able to be vaccinated.
In response to the pandemic, several adjuvanted and non-adjuvanted vaccines were developed. Oil-in-water emulsion adjuvants, such as AS03 and MF59, enhance the immune response to vaccine antigen and could reduce the dose of antigen needed. The use of an adjuvanted 3.75-μg dose of vaccine allows four times as much vaccine to be available compared to the standard influenza vaccine, which contains 15 μg hemagglutinin antigen (HA). As pandemic vaccine supplies were limited, the WHO suggested development of adjuvanted vaccines [10]. In the Republic of Korea, MF59-adjuvanted vaccine containing 3.75 μg HA was chosen to be administered during the vaccination campaign. Despite the novel MF59 adjuvant vaccine being licensed for human use a decade earlier, the mechanism of action is still unknown. Recent studies have shown that the MF59 adjuvant is a strong inducer of cytokines, cytokine receptors, and genes involved in leukocyte migration and antigen presentation [11, 12].
Although a single 3.75-μg dose of MF59-adjuvanted vaccine has shown good immunogenicity in young adults [7, 13], its immunogenicity in dialysis patients has not yet been investigated. Dialysis patients are considered immunocompromised and at increased risk of infection. However, immune dysfunction in dialysis patients might also compromise vaccine response. In a recent study, a 3.75-μg dose of MF59-adjuvanted vaccine was not satisfactory in the elderly who are also supposed to have a decreased immune response [13].
In this study, we evaluated the immunogenicity of a single 3.75-μg dose of MF59-adjuvanted 2009 influenza A/H1N1 vaccine in dialysis patients and compared the immune response between hemodialysis (HD) and peritoneal dialysis (PD) patients.
Materials and methods
Study design
From January to June 2010, we conducted a single-center, prospective study at our university hospital. Dialysis patients scheduled to receive pandemic H1N1 influenza vaccine were enrolled after providing an informed consent. Patients under maintenance HD or PD, aged >18 years were included. Exclusion criteria were pregnancy, patients under acute dialysis or combined HD and PD therapy, current febrile illness or another acute illness, history of allergic reaction to influenza vaccine or egg derivatives, and history of infection with A/H1N1 2009 virus.
All participants received a single 3.75-μg dose of MF59-adjuvanted vaccine by intramuscular injection into the deltoid muscle. For immunogenicity assays, serum samples were obtained before vaccination (day 0) and 28 days after vaccination (day 28). Clinical and laboratory data were extracted from patient medical records at baseline. All laboratory data including serum creatinine in HD patients were measured through blood sampling immediately before initiation of one hemodialysis session with overnight fasting. In assessment of dialysis adequacy, weekly standardized Kt/V in HD patients was calculated by equation of Leypoldt [14] and weekly Kt/V in PD patients was estimated using the standard method to measure total weekly urea [15].
Vaccines
The influenza A/H1N1 vaccine, a monovalent MF59-adjuvanted (Novartis, Marburg, Germany) inactivated split-virus vaccine, was produced by Green Cross Corporation (Yong-in, Korea). Each vaccine (0.25 mL) contained 3.75 μg of HA derived from A/California/7/2009 NYMC X-179A, 4.88 mg of squalene MF-59, 0.59 mg of polysorbate 80, and 0.59 mg of sorbitan trioleate in buffer. The vaccine was prepared in embryonated chicken eggs with the standard techniques that are used for the production of seasonal trivalent inactivated vaccine.
Immunogenicity assessment
All sera were tested by hemagglutination inhibition (HI) assays according to the WHO standard methods with the use of turkey red blood cells (TCM Bio Inc., Seoul, Korea) [6, 16]. Non-specific inhibitors were removed from serum by overnight treatment with a receptor destroying enzyme, and then non-specific agglutinins were removed by incubation with packed turkey red blood cells. A starting dilution of serum for HI assay was 1:10, and antibody titers below the lower limit of 1:10 were estimated to be a value of 1:5.
The three co-primary endpoints were defined as—seroprotection rate, the proportion of participants achieving antibody titer ≥1:40 after vaccination; seroconversion rate, the proportion of participants with a pre-vaccination antibody titer <1:10 and a post-vaccination titer ≥1:40, or a pre-vaccination titer ≥1:10 and an increase in the titer by a factor of four or more; or geometric mean titer (GMT) ratio, the ratio of the GMT after vaccination to the GMT before vaccination. Immunogenicity was assessed by the guidelines of the Committee for Proprietary Medicinal Products (CPMP) of the European Medical Agency [17]. According to the guidelines, one of the following criteria should be met to be beneficial—a seroprotection rate >70 %, or a seroconversion rate >40 %, or a GMT ratio >2.5 for young subjects (aged 18–60 years); a seroprotection rate >60 %, or a seroconversion rate >30 %, or a GMT ratio >2.0 for elderly subjects (aged >60 years).
Statistical analysis
Demographic variables are presented as mean ± SD. To compare HI titers of pre-vaccination and post-vaccination among the groups, the Kruskal–Wallis test was performed. The Wilcoxon test was used to compare HI titers obtained before and after vaccination. Non-parametric dependent variables between the groups were compared by Mann–Whitney U test. Categorical variables were analyzed using the Chi-squared test or Fisher’s exact test. A logistic regression analyses were used to assess the associations of baseline characteristics and antibody responses. All statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) and a P value <0.05 was considered statistically significant.
Results
Study participants
51 HD patients and 35 PD patients were enrolled. They received a single 3.75-μg dose of MF-59-adjuvanted vaccine; however, 3 and 1 patient from each group were lost to follow-up or were not available for their second antibody titer. Overall, 82 dialysis patients (48 HD patients and 34 PD patients, respectively) were included in immunogenicity analysis.
Baseline characteristics
The baseline characteristics are shown in Table 1. The HD group was older (mean age 58.0 ± 12.0 vs. 50.1 ± 11.6, P = 0.01) and had more co-morbid conditions than the PD group (39.6 vs. 17.6 %, P = 0.034). Sex, underlying diabetes, and dialysis duration were comparable between the groups. The number of patients with inadequate dialysis, defined as weekly standardized Kt/V <2.0 in HD and weekly Kt/V <1.7 in PD, was also comparable between the groups. 14 HD patients (29.2 %) and 3 PD patients (8.8 %) had received the seasonal trivalent influenza vaccine in winter 2009 (P = 0.025). There was no significant relationship between prevalence of seasonal vaccination and socio-economic status. Immunosuppressant drugs were being taken by 3 (6.3 %) and 1 (2.9 %) patient from each group.
Immunogenicity
The results of the immunogenicity assessment are shown in Table 2. Among HD patients, 4 of 29 young patients (13.8 %) and 1 of 19 elderly patients (5.3 %) had HI titers of ≥1:40 at baseline, with no significant difference between young and elderly patients. The baseline GMTs were comparable between young and elderly patients. Four weeks after vaccination, the GMTs were significantly increased compared with the baseline GMTs and did not differ between young and elderly patients (51.2 ± 51.4 vs. 14.1 ± 20.7 in young patients, P = 0.012; 37.9 ± 73.9 vs. 6.8 ± 8.0 in elderly patients, P = 0.018, respectively). The rates of seroprotection and seroconversion (27.6 and 17.2 % in young patients and 31.6 and 26.3 % in elderly patients, respectively) did not meet the CPMP criteria. However, the GMT ratio met the CPMP criteria in both young and elderly patients (5.1 ± 12.2 in young patients, 6.8 ± 14.7 in elderly patients, respectively). Post-vaccination GMTs and three co-primary endpoints (GMT ratio, seroprotection rate, seroconversion rate) showed no significant difference between young and elderly patients.
In PD patients, none of 25 young patients (0.0 %) and 1 of 9 elderly patients (11.1 %) had HI titers of ≥1:40 at baseline, and did not differ between young and elderly patients. The baseline GMTs were comparable between young and elderly patients. Among PD patients, GMTs were increased only in young patients (39.8 ± 51.4 vs. 6.8 ± 5.0 in young patients, P = 0.001; 13.3 ± 25.0 vs. 13.3 ± 25.0 in elderly patients, P = 1.000, respectively). The rates of seroprotection and seroconversion (36.0 and 36.0 % in young patients and 11.1 and 0.0 % in elderly patients, respectively) did not meet the CPMP criteria. The GMT ratio met the CPMP criteria only in young patients, and was significantly higher than that of elderly patients (6.0 ± 8.6 vs. 1.0 ± 0.0, P = 0.006).
Comparison of immunogenicity between dialysis groups
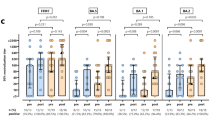
At baseline, both the GMTs and rates of HI titers of ≥1:40 showed no significant difference between dialysis groups regardless of ages. Among young patients, both weekly standardized Kt/V in HD patients and weekly Kt/V in PD patients were 2.1 ± 0.2 and 2.4 ± 0.8, respectively. The number of patients with inadequate dialysis were comparable between dialysis groups [2 (6.9 %) vs. 4 (16 %), P = 0.399]. There were significant differences in age, glucose, total protein, albumin between HD and PD patients (50.2 ± 7.6 vs. 45.2 ± 9.3 in age, P = 0.041; 106.4 ± 49.5 vs. 148.6 ± 89.1 in glucose, P = 0.008; 6.9 ± 0.5 vs. 6.4 ± 0.6 in total protein, P = 0.008; and 4.0 ± 0.3 vs. 3.8 ± 0.3 in albumin, P = 0.004, respectively). After vaccination in young patients as shown in Fig. 1a–c, three co-primary endpoints were comparable between dialysis groups. Among elderly patients, both weekly standardized Kt/V in HD patients and weekly Kt/V in PD patients were 2.1 ± 0.2 and 2.7 ± 1.0, respectively. The number of patients with inadequate dialysis were comparable between dialysis groups [4 (21.1 %) vs. 2 (22.2 %), P = 0.399]. Age was significantly lower in PD patients than in HD patients (69.9 ± 6.4 vs. 63.8 ± 3.3, P = 0.041). Although other variables including glucose, total protein, and albumin showed no significant differences between dialysis groups, three co-primary endpoints were relatively low in PD patients compared to HD patients (Fig. 1d–f).
Comparison of antibody responses between hemodialysis (HD) and peritoneal dialysis (PD) patients. a Seroprotection rate, b seroconversion rate, and c geometric mean titer ratio in subjects aged 18–60 years. d Seroprotection rate, e seroconversion rate, and f geometric mean titer ratio in subjects aged >60 years
Analysis of predictive factors for immune response
Dialysis patients who developed seroconversion were considered as immune responders. In univariate analysis, baseline GMTs, age, sex, dialysis duration, underlying diabetes, comorbidity, previous seasonal vaccination, use of immunosuppressants, laboratory variables including blood urea nitrogen, creatinine, glucose, total protein, albumin, hemoglobin, dialysis modality, dose of dialysis measured by weekly standardized Kt/V in HD and weekly Kt/V in PD, and number of patients with inadequate dialysis were not related to immune response. Similarly, in multivariate analysis, none of them was independently associated with the immune response.
Discussion
Dialysis patients are immunocompromised and recognized as a high risk group for serious influenza-related complications. Uremia-associated multiple immunologic abnormalities, including defects in complement activation, neutrophil function, and both B-cell and T-cell function, have been described in dialysis patients [18–20]. As a result, although international recommendations differed slightly across organizations, dialysis patients were consistently included in the priority groups for pandemic vaccination [21]. However, immune dysfunction in dialysis patients might also compromise vaccine response. Nevertheless, little is known about whether current vaccination strategy would provide sufficient immunogenicity for dialysis patients.
To the best of our knowledge, this is the first prospective cohort study on the immunogenicity induced by a single 3.75-μg HA MF59-adjuvanted 2009 A/H1N1 vaccine in dialysis patients. In a previous study with MF59-adjuvanted pandemic vaccine, the rates of seroprotection and seroconversion were excellent (92 and 88 %, respectively) in young adults aged 18–50 years even at a single 3.75-μg HA concentration [7]. Recently, Cheong et al. [13] also reported sufficient antibody response (78 % seroprotection, 67 % seroconversion) in young adults aged 18–64 years following a single 3.75-μg dose of MF59-adjuvanted vaccine. However, in elderly subjects aged >65 years who are considered as immunocompromised, a single 3.75-μg dose of MF59-adjuvanted vaccine induced a suboptimal antibody response (44 % seroprotection, 37 % seroconversion). Elderly subjects required a higher HA concentration (7.5 μg HA) or a two-dose schedule to elicit a sufficient antibody response.
In this study, the GMTs were significantly increased after vaccination in the majority of dialysis patients. However, both the rates of seroprotection (range 11−36 %) and seroconversion (range 0−36 %) were not sufficient to meet the CPMP criteria [17]. These results are different to those of previous studies, showing a rate of 78–92 % seroprotection and 67–88 % seroconversion in healthy young adults [7, 13]. Our results are similar or even lower to those of healthy elderly subjects [13]. Considering an HI titer of ≥40 is associated with a ≥50 % reduction in the risk of influenza virus infection or disease in susceptible populations [22, 23], our findings suggest that a single 3.75-μg dose of MF59-adjuvanted vaccine is suboptimal to elicit protective antibody response in dialysis patients.
It is well known that dialysis patients are associated with decreased immune response to several vaccines. When the hepatitis B vaccine is used in an immunocompetent population using a three-dose schedule, a 90–95 % seroprotection rate is expected. However, in dialysis patients, seroprotection rates are substantially low (50–60 %) and anti-HB levels decline more rapidly [24, 25]. To improve the immune responses, current guidelines recommend higher doses of vaccine than the general population [26]. Unlike research on hepatitis B vaccine, it is difficult to interpret seasonal influenza vaccine studies in dialysis patients. In several studies, dialysis patients could mount an adequate antibody response similar to healthy controls [27, 28], whereas in other studies, dialysis patients showed a poorer response than the general population [29, 30]. Current guidelines recommend influenza vaccination every year for dialysis patients [31, 32]; however, frequent previous vaccinations can influence the immunogenicity. Furthermore, annual reformulation of vaccine using different strains also makes comparisons across the studies difficult. For these reasons, caution should be used in extrapolating results from seasonal influenza studies to the new pandemic vaccination.
Regarding the 2009 pandemic vaccine, Mulley et al. [33] have shown that a single 15-μg dose of non-adjuvanted vaccine, which contains four times as much HA as our vaccine, resulted in a decreased antibody response in hemodialysis patients compared with healthy controls—56 versus 87 % in seroprotection and 45 versus 77 % in seroconversion, respectively. Two recent studies conducted with a 3.75-μg dose of AS03-adjuvanted vaccine also demonstrated a decreased antibody response in dialysis patients. Labriola et al. [34] compared the seroconversion rates between hemodialysis patients and healthy controls. After a single dose of vaccine, seroconversion was observed in only 34 of 58 (64 %) hemodialysis patients, in contrast to 30 of 32 (94 %) healthy controls. Dikow et al. [35] compared the efficacy of a one-dose schedule and a two-dose schedule in hemodialysis patients. In their study, seroprotection was observed in 41 of 64 patients (64 %) with the one-dose schedule, which was lower than the predicted seroprotection rate (98 %) in healthy adults. In two previous studies with adjuvant vaccine, despite the antibody response being blunted in dialysis patients, a single 3.75-μg dose of AS03-adjuvanted vaccine was sufficient to provide clinical protection.
In our study, however, both the rates of seroconversion and seroprotection were suboptimal to confer clinical protection. These discrepancies may be due to the different adjuvants (MF59 vs. AS03) and assays (HI vs. ELISA). As there are no available data on comparing immunogenicity between adjuvants, it is not clear whether AS03 adjuvant is more immunogenic over MF59 adjuvant in dialysis patients. In our study, HI assay was applied for determination of antibody response. Although a recent study has demonstrated that microneutralization is more sensitive than traditional HI assay, HI assay is still considered as the primary test for assessment of pandemic influenza vaccine [36]. The use of standard HI assay could reduce interlaboratory variation and allows comparison of the results of our study with those of other H1N1 vaccine trials. Despite the differences in adjuvants and assays, pre-vaccination immune status may also contribute to the discrepancies. In previous studies conducted in Europe, antibody titers of ≥1:40 at baseline were reported in about 30 % of study subjects, similar to Australia and the USA [6, 37]. High rates of detectable antibodies at baseline suggest previous exposure or subclinical infection in those countries. In our study, HI titers of ≥1:40 at baseline were observed in 0–13 % of participants, which were consistent with the 4–12 % from the United Kingdom and China [7, 38]. Considering that serologic response might differ according to the degree of pre-vaccination immune status, the relative low antibody response in our study could be explained by the low rates of HI titers of ≥1:40 at baseline. Moreover, our results reflect a more precise immune response in dialysis patients who are immunologically naïve to new pandemic influenza strain. Indeed, in a more recent study by Crespo et al. [39], hemodialysis patients showed suboptimal seroconversion rates (33 %) after excluding subjects with HI titers of ≥1:40 at baseline.
Among PD patients, some of the differences between young and the elderly are interesting. Elderly patients showed a relatively lower GMT level than young patients. In fact, none of the elderly patients seroconverted. Older age is consistently associated with poorer response to vaccine both in the general population [7, 8, 13, 40] and dialysis patients [35, 39, 41]. Less vigorous antibody response in the elderly is associated with several factors such as a decrease in the number of Langerhans cells, the limited capacity of dendritic cells to present antigen, defects in the expression of Toll-like receptors, and reduced expression of MHC class I and II molecules [42]. As a result, nonresponse in the elderly PD patients is not surprising because the immune compromise from the elderly would compound the existing immune deficiency of uremia. Another interesting finding is different antibody response between dialysis modes. It has been suggested that the immune response to vaccine in PD patients are better than HD patients [43–45]. It is thought that better clearance of middle molecules by peritoneal dialysis is the cause. Indeed, several studies have shown better antibody response in PD patients compared with HD patients after seasonal influenza vaccination [30, 46, 47]. However, a recent meta-analysis with a mean age of subject cohorts ranging from 12.2 to 61.0 years demonstrated no significant differences between dialysis mode and seroresponse to hepatitis B virus vaccine in a dialysis population [48]. More recently, Lin et al. [49] reported that the decay rate of antibody titers in the PD group is faster than in the HD group after hepatitis B vaccination. In our study, antibody response was comparable in young patients between dialysis modes despite unfavorable conditions in PD patients (high serum creatinine and glucose level, low total protein and albumin level) except younger ages. However, in elderly patients, antibody response was relatively low in PD patients compared to HD patients even though the conditions of PD patients were favorable (younger ages) or similar to HD patients. These findings suggest that potential amelioration of immune response by PD may be blunted in the elderly and differ from young adults. To clarify why the antibody response is relatively weak in elderly PD patients as compared to elderly HD patients, further studies would be needed.
Our data showed that seasonal influenza vaccine does not influence the antibody response to pandemic vaccine. As the 2009 A/H1N1 strain is antigenetically distinct from seasonal H1N1 strains, seasonal influenza vaccine is not cross-reactive with the 2009 A/H1N1 virus in HI assays [50]. Underlying medical conditions such as diabetes mellitus and comorbidity have been suggested as a factor to nonresponse [51, 52]. However, in this study, such factors were not associated with nonresponse in addition to uremia.
Our study has some limitations. First, we included a relatively small number of patients, which may limit the generalizability of the results. Second, solicited local and systemic adverse events were not collected; however, no serious adverse events were reported. Adverse events are more common for the MF59-adjuvanted vaccine than for the non-adjuvanted vaccine, but are generally mild and transient [7, 13]. Third, long-term immunogenicity was not investigated. In addition to the blunted antibody response, antibody titers decline more rapidly in dialysis patients compared with healthy individuals. Thus, more studies on the long-term immunogenicity and clinical effectiveness after immunization are needed.
In summary, a single 3.75-μg dose of MF59-adjuvanted vaccine was suboptimal to elicit protective antibody response in dialysis patients. Immune response against vaccine was compromised especially in elderly PD patients. Trials of different vaccination protocols such as a two-dose schedule or a higher HA dose of MF59-adjuvanted vaccine are necessary for improving antibody response in dialysis patients.
References
World Health Organization. DG statement following the meeting of the emergency committee. http://www.who.int/csr/disease/swineflu/4thmeetingihr/en/index.html. Accessed 1 Sept 2011.
Nicoll A, Ammon A, Amato Gauci A, Ciancio B, Zucs P, Devaux I, et al. Experience and lessons from surveillance and studies of the 2009 pandemic in Europe. Public Health. 2010;124:14–23.
Marcelli D, Marelli C, Richards N. Influenza A(H1N1)v pandemic in the dialysis population: first wave results from an international survey. Nephrol Dial Transplant. 2009;24:3566–72.
Johansen K, Nicoll A, Ciancio BC, Kramarz P. Pandemic influenza A(H1N1) 2009 vaccines in the European Union. Euro Surveill. 2009;14:19361.
Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. New Engl J Med. 2006;354:1343–51.
Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13.
Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of influenza A (H1N1) 2009 monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–35.
Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–23.
Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03(A)-adjuvant: preliminary report of an observer-blind, randomized trial. Vaccine. 2010;28:1740–5.
World Health Organization. Influenza pandemic preparedness and response: report by the Secretariat. http://apps.who.int/gb/ebwha/pdf_files/EB115/B115_44-en.pdf. Accessed 1 Sept 2011.
Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA. 2008;105:10501–6.
Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27:3331–4.
Cheong HJ, Song JY, Heo JY, Noh JY, Choi WS, Park DW, et al. Immunogenicity and safety of the influenza A/H1N1 2009 inactivated split-virus vaccine in young and older adults: MF59-adjuvanted vaccine versus nonadjuvanted vaccine. Clin Vaccine Immunol. 2011;18:1358–64.
Leypoldt JK. Urea standard Kt/V for assessing dialysis treatment adequacy. Hemodial Int. 2004;8:193–7.
Nolph KD, Moore HL, Twardowski ZJ, Khanna R, Prowant B, Meyer M, et al. Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis. ASAIO J. 1992;38:M139–42.
World Health Organization. WHO manual on animal influenza diagnosis and surveillance. http://whqlibdoc.who.int/hq/2002/WHO_CDS_CSR_NCS_2002.5.pdf. Accessed 1 Sept 2011.
Committee for Proprietary Medicinal Products of the European Agency for the Evaluation of Medicinal Products. Note for guidance on harmonization of requirements for influenza vaccines. http://www.ema.europa.eu/pdfs/human/bwp/021496en.pdf. Accessed 1 Sept 2011.
Descamps-Latscha B. The immune system in end-stage renal disease. Curr Opin Nephrol Hypertens. 1993;2:883–91.
Descamps-Latscha B, Herbelin A. Long-term dialysis and cellular immunity: a critical survey. Kidney Int Suppl. 1993;41:S135–42.
Haag-Weber M, Horl WH. Uremia and infection: mechanisms of impaired cellular host defense. Nephron. 1993;63:125–31.
Hanquet G, Van Damme P, Brasseur D, De Cuyper X, Gregor S, Holmberg M, et al. Lessons learnt from pandemic A(H1N1) 2009 influenza vaccination. Highlights of a European workshop in Brussels (22 March 2010). Vaccine. 2011;29:370–7.
de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel). 2003;115:63–73.
Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond). 1972;70:767–77.
Chow KM, Law MC, Leung CB, Szeto CC, Li PK. Antibody response to hepatitis B vaccine in end-stage renal disease patients. Nephron Clin Pract. 2006;103:c89–93.
Ramezani A, Eslamifar A, Banifazl M, Ahmadi F, Maziar S, Razeghi E, et al. Efficacy and long-term immunogenicity of hepatitis B vaccine in haemodialysis patients. Int J Clin Pract. 2009;63:394–7.
Edey M, Barraclough K, Johnson DW. Review article: hepatitis B and dialysis. Nephrology (Carlton). 2010;152:137–45.
Song JY, Cheong HJ, Ha SH, Kee SY, Jeong HW, Kim WJ. Active influenza immunization in hemodialysis patients: comparison between single-dose and booster vaccination. Am J Nephrol. 2006;26:206–11.
Scharpé J, Peetermans WE, Vanwalleghem J, Maes B, Bammens B, Claes K, et al. Immunogenicity of a standard trivalent influenza vaccine in patients on long-term hemodialysis: an open-label trial. Am J Kidney Dis. 2009;54:77–85.
Antonen JA, Hannula PM, Pyhala R, Saha HH, Ala-Houhala IO. Pasternack AI. Adequate seroresponse to influenza vaccination in dialysis patients. Nephron. 2000;86:56-61.
Cavdar C, Sayan M, Sifil A, Artuk C, Yilmaz N, Bahar H, et al. The comparison of antibody response to influenza vaccination in continuous ambulatory peritoneal dialysis, hemodialysis and renal transplantation patients. Scand J Urol Nephrol. 2003;37:71–6.
Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al., Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the advisory committee on immunization practices (ACIP), 2007. MMWR Recomm Rep. 2007;56:1–54.
Rangel MC, Coronado VG, Euler GL, Strikas RA. Vaccine recommendations for patients on chronic dialysis. The Advisory Committee on Immunization Practices and the American Academy of Pediatrics. Semin Dial. 2000;13:101–7.
Mulley WR, Visvanathan K, Hurt AC, Brown FG, Polkinghorne KR, Mastorakos T, et al. Mycophenolate and lower graft function reduce the seroresponse of kidney transplant recipients to pandemic H1N1 vaccination. Kidney Int. 2012. doi:10.1038/ki.2012.106 (Epub ahead of print).
Labriola L, Hombrouck A, Maréchal C, Van Gucht S, Brochier B, Thomas I, et al. Immunogenicity of an adjuvanted 2009 pandemic influenza A (H1N1) vaccine in haemodialysed patients. Nephrol Dial Transplant. 2011;26:1424–8.
Dikow R, Eckerle I, Ksoll-Rudek D, Hampel H, Schwenger V, Zeier M, et al. Immunogenicity and efficacy in hemodialysis patients of an AS03(A)-adjuvanted vaccine for 2009 pandemic influenza A(H1N1): a nonrandomized trial. Am J Kidney Dis. 2011;57:716–23.
Veguilla V, Hancock K, Schiffer J, Gargiullo P, Lu X, Aranio D, et al. Sensitivity and specificity of serologic assays for detection of human infection with 2009 pandemic H1N1 virus in U.S. populations. J Clin Microbiol. 2011;49:2210–5.
Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–8.
Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66.
Crespo M, Collado S, Mir M, Cao H, Barbosa F, Serra C, et al. Efficacy of influenza A H1N1/2009 vaccine in hemodialysis and kidney transplant patients. Clin J Am Soc Nephrol. 2011;6:2208–14.
Cheong HJ, Song JY, Heo JY, Noh JY, Choi WS, Park DW, et al. Immunogenicity and safety of influenza A (H1N1) 2009 monovalent inactivated split vaccine in Korea. Vaccine. 2011;29:523–7.
Temiz G, Kasifoglu N, Kiris A, Ozturk S, Sahin G, Yalcin AU, et al. Immune response after a single vaccination against 2009 influenza A H1N1 in hemodialysis patients. Ren Fail. 2010;32:716–20.
Parodi V, de Florentiis D, Martini M, Ansaldi F. Inactivated influenza vaccines: recent progress and implications for the elderly. Drugs Aging. 2011;28:93–106.
Giacchino F, Quarello F, Pellerey M, Piccoli G. Continuous ambulatory peritoneal dialysis improves immunodeficiency in uremic patients. Nephron. 1983;35:209–10.
Popovich RP, Moncrief JW, Nolph KD, Ghods AJ, Twardoski ZJ, Pyle WK. Continuous ambulatory peritoneal dialysis. Ann Intern Med. 1978;88:448–52.
Touraine JL, Touraine F, Revillard JP, Brochier J, Traeger J. T lymphocytes and serum inhibitors of cell-mediated immunity in renal insufficiency. Nephron. 1975;14:195–208.
Versluis DJ, Beyer WE, Masurel N, Diderich PP, Kramer P, Weimar W. Intact humoral immune response in patients on continuous ambulatory peritoneal dialysis. Nephron. 1988;49:16–9.
Antonen JA, Hannula PM, Pyhälä R, Saha HH, Ala-Houhala IO, Pasternack AI. Adequate seroresponse to influenza vaccination in dialysis patients. Nephron. 2000;86:56–61.
Fabrizi F, Dixit V, Bunnapradist S, Martin P. Meta-analysis: the dialysis mode and immunological response to hepatitis B virus vaccine in dialysis population. Aliment Pharmacol Ther. 2006;23:1105–12.
Lin SY, Liu JH, Lin CC, Wang SM, Tsai CA, Chou CY, et al. Comparison of hepatitis B surface antibody decay rates after vaccination between hemodialysis and peritoneal dialysis patients. Vaccine. 2011;29:3738–41.
Deans GD, Stiver HG, McElhaney JE. Influenza vaccines provide diminished protection but are cost-saving in older adults. J Intern Med. 2010;267:220–7.
Nam JS, Kim AR, Yoon JC, Byun Y, Kim SA, Kim KR, et al. The humoral immune response to the inactivated influenza A (H1N1) 2009 monovalent vaccine in patients with type 2 diabetes mellitus in Korea. Diabet Med. 2011;28:815–7.
Fabrizi F, Dixit V, Martin P, Messa P. Meta-analysis: the impact of diabetes mellitus on the immunological response to hepatitis B virus vaccine in dialysis patients. Aliment Pharmacol Ther. 2011;33:815–21.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Son, J., Lee, S.B., Lee, D.W. et al. Immunogenicity of low-dose MF59-adjuvanted 2009 influenza A/H1N1 vaccine in dialysis patients. Clin Exp Nephrol 17, 275–283 (2013). https://doi.org/10.1007/s10157-012-0696-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-012-0696-1