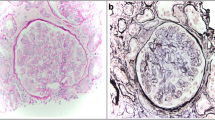
Abstract
A-52-year-old man presented to our hospital with nephrotic syndrome caused by membranous nephropathy. Early gastric adenocarcinoma confined to the submucosa, and well-differentiated adenocarcinoma in a sigmoid adenoma were detected by screening endoscopy. Two years after complete endoscopic resection of these tumors, the estimated 24-h urinary protein excretion decreased, and serum total protein and albumin returned to their normal levels although he had no immunosuppressive therapy. Thus, this case was considered to be a case of secondary membranous nephropathy to cancer, although whether the pathogenesis was due to circulating or in situ immune complexes is unknown. The suspected antigen component of this immune complex can include carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9). In our patient, however, serum CEA and CA19-9 were within normal values during the clinical course, and detected cancers were early stage and very small. Recently, the existence of anti-mucin 1 (MUC1) antibodies before carcinogenesis and their usefulness for early detection of cancer were reported. We tried to stain tumors and glomeruli for MUC1 but, although we had positive findings in both tumors but not in glomeruli, the role of MUC1 in the pathogenesis of membranous nephropathy is unknown. To the best of our knowledge, paraneoplastic nephrosis caused by double early cancers expressing MUC1 in the gastrointestinal tract has not been previously reported.




Similar content being viewed by others
References
Jefferson JJ, Couser WG. Therapy of membranous nephropathy associated with malignancy and secondary causes. Semin Nephrol. 2003;23:400–5.
Hoshino J, Hara S, Ubara Y, Takaya H, Suwabe T, Sawa N, et al. Distribution of IgG subclasses in a biopsy specimen showing membranous nephropathy with anti-glomerular basement membrane glomerulonephritis: an uncharacteristically good outcome with corticosteroid therapy. Am J Kidney Dis. 2005;45:e67–72.
Kim HO, Hwang SI, Yoo CH, Kim H. Preoperative colonoscopy for patients with gastric adenocarcinoma. J Gastroenterol Hepatol. 2009;24:1740–4.
Bae JS, Lee JH, Ryu KW, Kim YW, Bae JM. Characteristics of synchronous cancers in gastric cancer patients. Cancer Res Treat. 2006;38:25–9.
Pascal RR, Slovin SF. Tumor directed antibody and carcinoembryonic antigen in the glomeruli of a patient with gastric carcinoma. Hum Pathol. 1980;11:679–82.
Wakashin M, Wakashin Y, Iesato K, Ueda S, Mori Y, Tsuchida H, et al. Association of gastric cancer and nephrotic syndrome. An immunologic study in three patients. Gastroenterology. 1980;78:749–56.
Row PG, Cameron JS, Turner, Evans DJ, White RH, Ogg CS, et al. Membranous nephropathy. Long-term follow-up and association with neoplasia. Q J Med. 1975;44:207–39.
Couser WG, Wagonfeld JB, Spargo BH, Lewis EJ. Glomerular deposition of tumor antigen in membranous nephropathy associated with colonic carcinoma. Am J Med. 1974;57:962–70.
Braymann M, Thathiah A, Carson DD. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol. 2004;2:4.
Leroy X, Copin MC, Devisme L, Buisine MP, Aubert JP, Gosselin B, et al. Expression of human mucin genes in normal kidney and renal cell carcinoma. Histopathology. 2002;40:450–7.
Leroy X, Devisme L, Buisine MP, Copin MC, Aubert S, Gosselin B, et al. Expression of human mucin genes during normal and abnormal renal development. Am J Clin Pathol. 2003;120:544–50.
Pinheiro SP, Hankinson SE, Tworoger SS, Rosner BA, McKolanis JR, Finn OJ, et al. Anti-MUC1 antibodies and ovarian cancer risk: prospective data from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:1595–601.
Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennette EP, Mandel U, et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010;70:1306–13.
Acknowledgments
We are grateful to Dr. Masahisa Kyogoku for discussions on pathology and helpful suggestions.
Conflict of interest
This study received no financial support and none of the authors has any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Matsui, S., Tsuji, H., Takimoto, Y. et al. Clinical improvement of membranous nephropathy after endoscopic resection of double early gastrointestinal cancers. Clin Exp Nephrol 15, 285–288 (2011). https://doi.org/10.1007/s10157-010-0389-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-010-0389-6