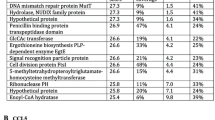
Abstract
The Mycobacterium avium complex (MAC) invades cultured human bronchial cells, can replicate intracellularly, and facilitates the release of inflammatory cytokines and chemokines from cells. The purpose of this study was to examine the effects of clarithromycin (CAM) on MAC invasion, replication, and the release of cytokines and chemokines. A human bronchial epithelial cell line (BEAS-2B) monolayer grown on a tissue culture plate was infected with MAC. After 24 h, the cells were washed with Hanks’ buffered salt solution, and extracellular bacteria were killed. The monolayer was further cultured for 5 days in medium containing CAM and subjected to a replication assay. The supernatants were assessed using a microchemotaxis assay and enzyme-linked immunosorbent assay (ELISA). mRNA expression was evaluated using a DNA array. The amount of intracellular MAC on day 5 of culture was significantly lower in the presence of CAM at the levels of 1× and 4× MIC. CAM inhibited the release of chemotactic activity and the production of interleukin (IL)-8 and macrophage chemotactic protein (MCP)-1. DNA array analysis of mRNA expression in BEAS-2B cells showed that CAM inhibited the expression of inflammatory cytokines and chemokines, involving IL-6, MCP-1, and IL-8 mRNA. MAC invaded and replicated in BEAS-2B cells and induced the production of chemotactic factors. In contrast, CAM may have bactericidal and bacteriostatic effects leading to the inhibition of inflammatory events.

Similar content being viewed by others
Abbreviations
- MAC:
-
Mycobacterium avium complex
- CAM:
-
Clarithromycin
- CFU:
-
Colony-forming unit
- NCA:
-
Neutrophil chemotactic activity
- MCA:
-
Monocyte chemotactic activity
References
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:744–5.
Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–8.
Yamazaki Y, Kubo K, Takamizawa A, Yamamoto H, Honda T, Sone S. Markers indicating deterioration of pulmonary Mycobacterium avium-intracellulare infection. Am J Respir Crit Care Med. 1999;160:1851–5.
Desaki M, Okazaki H, Sunazuka T, Omura S, Yamamoto K, Takizawa H. Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: possible role in the signaling pathway that regulates nuclear factor-kappaB activation. Antimicrob Agents Chemother. 2004;48:1581–5.
Fujita J, Ohtsuki Y, Suemitsu I, Shigeto E, Yamadori I, Obayashi Y, et al. Pathological and radiological changes in resected lung specimens in Mycobacterium avium intracellulare complex disease. Eur Respir J. 1999;13:535–40.
Tanaka E, Kimoto T, Tsuyuguchi K, Watanabe I, Matsumoto H, Niimi A, et al. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1999;160:866–72.
Wallace RJ Jr, Brown BA, Griffith DA, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153:1766–72.
Yamazaki Y, Danelishvili L, Wu M, Hidaka E, Katsuyama T, Stang B, et al. The ability to form biofilm influences Mycobacterium avium invasion and translocation of bronchial epithelial cells. Cell Microbiol. 2006;8:806–14.
Ashitani J, Mukae H, Hiratsuka T, Nakazato M, Kumamoto K, Matsukura S. Plasma and BAL fluid concentrations of antimicrobial peptides in patients with Mycobacterium avium-intracellulare infection. Chest. 2001;119:1131–7.
Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 1968;97:77–89.
Koyama S, Sato E, Nomura H, Kubo K, Miura M, Yamashita T, et al. The potential of various lipopolysaccharides to release monocyte chemotactic activity from lung epithelial cell and fibroblasts. Eur Respir J. 1999;14:545–52.
Falk W, Goodwin RH Jr, Leonard EJ. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–47.
Kobashi Y, Matsushima T. The effect of combined therapy according to the guidelines for the treatment of Mycobacterium avium complex pulmonary disease. Intern Med. 2003;42:670–5.
Ishiguro M, Koga H, Kohno S, Hayashi T, Yamaguchi K, Hirota M. Penetration of macrolides into human polymorphonuclear leukocytes. J Antimicrob Chemother. 1989;24:719–29.
Kunishima H, Takemura H, Yamamoto H, Kanemitsu K, Shimada J. Evaluation of the activity of antimicrobial agents against Legionella pneumophila multiplying in a human monocytic cell line, THP-a, and an alveolar epithelial cell line, A549. J Infect Chemother. 2000;6:206–10.
Hasegawa N, Nishimura T, Watabnabe M, Tasaka S, Nakano Y, Yamazaki K, et al. Concentrations of clarithromycin and active metabolite in the epithelial lining fluid of patients with Mycobacterium avium complex pulmonary disease. Pulm Pharmacol Ther. 2009;22:190–3.
Matsuyama W, Mizoguchi A, Iwami F, Koreeda Y, Wakimoto J, Kanazawa H, et al. Clinical investigation of pulmonary Mycobacterium avium complex infection in human T lymphotrophic virus type I carriers. Thorax. 2000;55:388–92.
Yamazaki Y, Kubo K, Sekiguchi M. Analysis of BAL fluid in M. avium-intracellulare infection in individuals without predisposing lung disease. Eur Respir J. 1998;11:1227–31.
Schultz MJ. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother. 2004;54:21–8.
Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, et al. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–8.
Griffith DE, Brown BA, Murphy DT, Girard WM, Couch L, Wallace RJ Jr. Initial (6-month) results of three-times-weekly azithromycin in treatment regimens for Mycobacterium avium complex lung disease in human immunodeficiency virus-negative patients. J Infect Dis. 1998;178:121–6.
Kohyama T, Takizawa H, Kawasaki S, Akiyama N, Sato M, Ito K. Fourteen-member macrolides inhibit interleukin-8 release by human eosinophilus from atopic donors. Antimicrob Agents Chemother. 1999;43:907–11.
Suzuki H, Shimomura A, Ikeda K, Furukawa M, Oshima T, Takasaka T. Inhibitory effect of macrolide on interleukin-8 secretion from cultured human nasal epithelial cells. Laryngoscope. 1997;107:1661–6.
Takizawa H, Desaki M, Ohtoshi T, Kawasaki S, Kohyama T, Sato M, et al. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;156:266–71.
Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43.
Saige H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, et al. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol. 2008;181:8521–7.
Acknowledgments
This study was supported by a Grant-in-Aid for AIDS Research from the Ministry of Health, Labor, and Welfare.
Conflicts of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yamazaki, Y., Ushiki, A., Tanabe, Y. et al. Antimicrobial and anti-inflammatory effects of clarithromycin on Mycobacterium avium complex replication in cultured human bronchial epithelial cells. J Infect Chemother 18, 683–688 (2012). https://doi.org/10.1007/s10156-012-0395-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-012-0395-6