Abstract
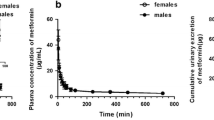
In this study, the involvement of sulfate conjugation and drug efflux transporter multidrug resistance-associated protein 2 (Mrp2) in sex-related differences in the pharmacokinetics of a new quinolone antimicrobial agent, garenoxacin, was investigated in Sprague–Dawley (SD) rats and Eisai hyperbilirubinemic rats (EHBRs) lacking Mrp2. The disappearance of garenoxacin from plasma in female SD rats was significantly faster than that in male SD rats after a single intravenous injection of garenoxacin (5 mg/kg). The systemic clearance of garenoxacin in female rats was approximately threefold larger than that of male rats (2.43 ± 0.31 and 0.87 ± 0.06 l/h/kg, respectively), suggesting the existence of sex-related differences in the pharmacokinetics of garenoxacin. When rats received a constant-rate infusion of garenoxacin, the contribution of biliary and renal excretion of garenoxacin was small, and no significant difference in the biliary (CLBILE) clearance of garenoxacin was observed between male and female SD rats. The metabolic clearance [CLM (SULF)] of garenoxacin to garenoxacin sulfate conjugate (which is mainly excreted into the bile) in female SD rats was 8.5-fold larger than that in male SD rats (27.9 ± 2.94 and 3.28 ± 0.07 ml/h/kg, respectively). The CLBILE of garenoxacin was decreased in male and female EHBRs by approximately 50% compared with that in male and female SD rats. These results suggest that sulfate conjugation, but not Mrp2, is mainly involved in the sex-related differences in the pharmacokinetics of garenoxacin.



Similar content being viewed by others
References
Hayakawa H, Fukushima Y, Kato H, Fukumoto H, Kadota T, Yamamoto H, et al. Metabolism and disposition of novel des-fluoro quinolone garenoxacin in experimental animals and interspecies scaling of pharmacokinetic parameters. Drug Metab Dispos. 2003;31:1409–18.
Kato H, Hayakawa H, Fukushima Y, Kadota T, Fukumoto H, Todo Y. Pharmacokinetics of garenoxacin in laboratory animal species. Jpn J Chemother. 2007;55(S-1):78–86.
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–8.
Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood–brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–24.
Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smith JJ, et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci USA. 1997;94:4028–32.
Oude Elferink RP, Meijer DK, Kuipers F, Jansen PL, Groen AK, Groothuis GM. Hepatobiliary secretion of organic compounds; molecular mechanisms of membrane transport. Biochim Biophys Acta. 1995;1241:215–68.
Cormet-Boyaka E, Huneau JF, Mordrelle A, Boyaka PN, Carbon C, Rubinstein E, et al. Secretion of sparfloxacin from the human intestinal Caco-2 cell line is altered by P-glycoprotein inhibitors. Antimicrob Agents Chemother. 1998;42:2607–11.
Murata M, Tamai I, Kato H, Nagata O, Kato H, Tsuji A. Efflux transport of a new quinolone antimicrobial agent, HSR-903, across the blood–brain barrier. J Pharmacol Exp Ther. 1999;290:51–7.
De Lange ECM, Marchand S, van den Berg DJ, van der Sandt ICJ, de Boer A, Delon GA, et al. In vitro and in vivo investigations on fluoroquinolones: effects of the P-glycoprotein efflux transporter on brain distribution of sparfloxacin. Eur J Pharm Sci. 2000;12:85–95.
Tamai I, Yamashita J, Kido Y, Ohnari A, Sai Y, Shima Y, et al. Limited distribution of new quinolone antimicrobial agents into brain caused by multiple efflux transporters at the blood–brain barrier. J Pharmacol Exp Ther. 2000;295:146–52.
Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, Suzuki N, et al. Active intestinal secretion of new quinolone antimicrobials and the partial contribution of P-glycoprotein. J Pharm Pharmacol. 2001;53:699–709.
Yamaguchi H, Yano I, Saito H, Inui K. Pharmacokinetic role of P-glycoprotein in oral bioavailability and intestinal secretion of grepafloxacin in vivo. J Pharmacol Exp Ther. 2002;300:1063–9.
Zhao YL, Cai SH, Wang L, Kitaichi K, Tatsumi Y, Nadai M, et al. Possible involvement of P-glycoprotein in the biliary excretion of grepafloxacin. Clin Exp Pharmacol Physiol. 2002;29:167–72.
Shimizu A, Miyoshi M, Sugie M, Ueyama J, Yamaguchi T, Sasaki T, et al. Possible involvement of P-glycoprotein in renal excretion of pazufloxacin in rats. Eur J Pharmacol. 2004;501:151–9.
Sasabe H, Tsuji A, Sugiyama Y. Carrier-mediated mechanism for the biliary excretion of the quinolone antibiotic grepafloxacin and its glucuronide in rats. J Pharmacol Exp Ther. 1998;284:1033–9.
Takenaka O, Horie T, Kobayashi K, Suzuki H, Sugiyama Y. Kinetic analysis of hepatobiliary transport for conjugated metabolites in the perfused liver of mutant rats (EHBR) with hereditary conjugated hyperbilirubinemia. Pharm Res. 1995;12:1746–55.
Yamazaki K, Mikami T, Hosokawa S, Tagaya O, Nozaki Y, Kawaguchi A, et al. A new mutant rat with hyperbilirubinemia (hyb). J Hered. 1995;86:314–7.
Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992;17:463–8.
Ito K, Suzuki H, Hirohashi T, Kume K, Shimizu T, Sugiyama Y. Molecular cloning of canalicular multispecific organic anion transporter defective in EHBR. Am J Physiol. 1997;272:G16–22.
Paulusma CC, Oude Elferink RP. The canalicular multispecific organic anion transporter and conjugated hyperbilirubinemia in rat and man. J Mol Med. 1997;75:420–8.
Johnson DR, Guo GL, Klaassen CD. Expression of rat multidrug resistance protein 2 (Mrp2) in male and female rats during normal and pregnenolone-16α-carbonitrile (PCN)-induced postnatal ontogeny. Toxicology. 2002;178:209–19.
Suzuki T, Zhao YL, Nadai M, Naruhashi K, Shimizu A, Takagi K, et al. Gender-related differences in expression and function of hepatic P-glycoprotein and multidrug resistance-associated protein (Mrp2) in rats. Life Sci. 2006;79:455–61.
Acknowledgments
We are extremely grateful to Toyama Chemical Co., Ltd. (Toyama, Japan) for its generous contribution of drugs. This work was supported in part by a grant-in-aid for scientific research (20590587) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hayashi, T., Abe, F., Kato, M. et al. Involvement of sulfate conjugation and multidrug resistance-associated protein 2 (Mrp2) in sex-related differences in the pharmacokinetics of garenoxacin in rats. J Infect Chemother 17, 24–29 (2011). https://doi.org/10.1007/s10156-010-0095-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-010-0095-z